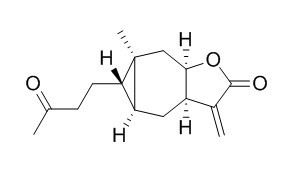
Carabrone
Some of carabrone derivatives exhibit antifungal activities in vitro or in vivo, the compounds with a pyridinyl residue can either efficiently inhibit spore germination or efficiently inhibit hyphal growth of B. cinerea.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Nutr.2021, 8: 687851.
Current Enzyme Inhibition2023, 19(1):55-64(10)
Journal of Medical Sciences2024, 44(5):p 222-227.
Phytomedicine.2024, 155760.
Front Nutr.2024, 11:1409309.
Front Pharmacol.2025, 16:1611342.
Applied Biological Chem. 2020, 26(63).
Molecules.2024, 29(23):5632.
Korean Journal of Plant Resources2021, 34(1):52-58.
iScience.2024, 4790628.
Related and Featured Products
Chem Biodivers. 2014 Jun;11(6):886-903.
Semisynthesis and antifungal activity of novel oxime ester derivatives of carabrone modified at C(4) against Botrytis cinerea.[Pubmed:
24934674]
METHODS AND RESULTS:
To continuously improve the potential utility of the natural lead compound of Carabrone in agrochemistry, Carabrone oxime and 36 novel oxime ester derivatives of Carabrone modified at C(4) were synthesized, and evaluated for their antifungal activities against Botrytis cinerea in vitro and in vivo.
CONCLUSIONS:
Of these 36 oxime ester derivatives, some compounds exhibited antifungal activities in vitro or in vivo.
It was found that compounds with a pyridinyl residue can either efficiently inhibit spore germination or efficiently inhibit hyphal growth of B. cinerea, and compound 9 exhibited the highest activity in vitro and in vivo with IC50 and EC50 values of 1.17 and 12.9 μg/ml, respectively. Further, the structure-activity relationships are also discussed.
Int J Mol Sci. 2014 Mar 11;15(3):4257-72.
Synthesis, antifungal activities and qualitative structure activity relationship of carabrone hydrazone derivatives as potential antifungal agents.[Pubmed:
24619221]
METHODS AND RESULTS:
Aimed at developing novel fungicides for relieving the ever-increasing pressure of agricultural production caused by phytopathogenic fungi, 28 new hydrazone derivatives of Carabrone, a natural bioactive sesquisterpene, in three types were designed, synthesized and their antifungal activities against Botrytis cinerea and Colletotrichum lagenarium were evaluated.
CONCLUSIONS:
The result revealed that all the derivatives synthesized exhibited considerable antifungal activities in vitro and in vivo, which led to the improved activities for Carabrone and its analogues and further confirmed their potential as antifungal agents.
Molecules. 2010 Sep 16;15(9):6485-92.
Synthesis and antifungal activity of carabrone derivatives.[Pubmed:
20877238]
METHODS AND RESULTS:
Nine derivatives 6-14 of Carabrone (1) were synthesized and tested in vitro against Colletotrichum lagenarium Ell et Halst using the spore germination method. Among all of the derivatives, compounds 6-8 and 12 showed more potent antifungal activity than 1.
CONCLUSIONS:
Structure-activity relationships (SAR) demonstrated that the γ-lactone was necessary for the antifungal activity of 1, and the substituents on the C-4 position of 1 could significantly affect the antifungal activity.
Planta Med. 2012 Jun;78(10):1002-9.
Sesquiterpene lactones from Inula hupehensis inhibit nitric oxide production in RAW264.7 macrophages.[Pubmed:
22648378]
METHODS AND RESULTS:
Phytochemical investigation of the aerial parts of Inula hupehensis Ling. led to the isolation and identification of 27 sesquiterpene lactones (1-27), including three new eudesmanolides (3-5), three new germacranolides (9-11), one new xanthanolide (16), two new Carabrone derivatives (25-26), and 18 known sesquiterpene lactones. The structures were elucidated by extensive spectroscopic methods and comparison to previously reported spectroscopic data.
CONCLUSIONS:
All compounds were evaluated for their inhibitory effects against LPS-induced nitric oxide production in RAW264.7 macrophages, and compound 5 showed the strongest activity with the IC₅₀ value of 3.2 ± 0.4 µM.