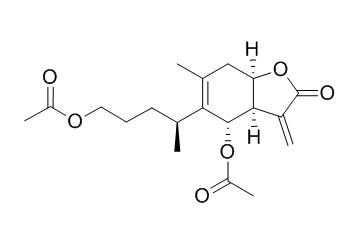
1,6-O,O-Diacetylbritannilactone
1,6-O,O-Diacetylbritannilactone(OODBL) has anti-inflammatory activity, it has a potential therapeutic efficacy on inflammatory diseases especially allergic airway disease as a lead compound. OODBL has anti-asthmatic activity, it reduces leukotriene C4 production and degranulation through the suppression of cytosolic phospholipase A2 phosphorylation and phospholipase Cγ-mediated Ca2+ influx in IgE/antigen-stimulated BMMCs. OODBL also exhibits potent antitumor activity against several human cancer cell lines.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Ethnopharmacol.2021, 267:113615.
J Colloid Interface Sci.2022, 622:298-308.
Korean J. Food Preserv. 2021, 28(6):846-856.
Pharmaceuticals (Basel).2024, 18(1):19.
Pharmacogn Mag.2015, 11(43):562-6
Agronomy2020, 10(3),388.
Bull.Natl.Mus.Nat.Sci.,Ser.B.2024, 50(2):79�C86
Eur J Pharmacol.2021, 906:174220.
Int J Mol Sci.2021, 22(2):770.
Nutr Cancer.2023, 75(1):376-387.
Related and Featured Products
Inflammation. 2017 Dec;40(6):1967-1974
1,6-O,O-Diacetylbritannilactone Inhibits Eotaxin-1 and ALOX15 Expression Through Inactivation of STAT6 in A549 Cells.[Pubmed:
28770377]
1,6-O,O-Diacetylbritannilactone (OODBL), a plant sesquiterpene lactone, was previously reported to show multiple pharmacological effects such as anti-cancer and anti-inflammatory activities.
METHODS AND RESULTS:
In this study, we investigated the anti-inflammatory effect of OODBL on interleukin (IL)-4-induced signal transducer and activator of transcription 6 (STAT6) signaling pathway in human lung A549 cells. We found that OODBL dramatically inhibited IL-4-induced messenger RNA (mRNA) expression of eotaxin-1 and arachidonate 15-lipoxygenase-1 (ALOX15) in a dose-dependent manner. To clarify the action mechanism of OODBL, we examined the effect of OODBL on activation of STAT6. OODBL decreased both STAT6 phosphorylation and reporter gene activity. Furthermore, OODBL suppressed phosphorylation of Janus Kinase 3 (JAK3) without affecting JAK1.
CONCLUSIONS:
Taken together, OODBL abolished IL-4-induced eotaxin-1 and ALOX15 mRNA expressions by repressing the activation of STAT6 and JAK3. These results suggest that OODBL has a potential therapeutic efficacy on inflammatory diseases especially allergic airway disease as a lead compound.
Nat Prod Commun. 2016 Jan;11(1):7-10.
Cytotoxic and Pro-apoptotic Activities of Sesquiterpene Lactones from Inula britannica.[Pubmed:
26996005]
METHODS AND RESULTS:
In this study, five known sesquiterpene lactones (STL) with an α-methylene-γ-lactone motif, including two eudesmanolides, 1β-hydroxyalantolactone (1) and ivangustin (2), and three 1,10-seco-eudesmanolides, 1-O-acetylbritannilactone (3), 1,6-O,O-Diacetylbritannilactone (4), and 6α-O-(2- methylbutyryl)britannilactone (5) were isolated from the flower heads of the medicinal plant Inula britannica.
Their structures were characterized by spectroscopic methods. X-ray data of 2 is reported for the first time.
CONCLUSIONS:
Among them, eudesmanolides 1 and 2 exhibited remarkable cytotoxicity against HEp2, SGC-7901 and HCT116 human cancer cell lines, comparable with etoposide (Vp-16) used as reference drug. Furthermore, treatment of HEp2 cells with 1 induced apoptosis associated with cleaved procaspase-3 and PARP.
The biological assays carried out with normal cells (CHO) revealed that all sesquiterpenes were weakly selective against the cancer cell lines tested.
Mol Nutr Food Res. 2007 Feb;51(2):229-38.
Involvement of MAPK, Bcl-2 family, cytochrome c, and caspases in induction of apoptosis by 1,6-O,O-diacetylbritannilactone in human leukemia cells.[Pubmed:
17262884]
1,6-O,O-Diacetylbritannilactone (OODBL) isolated from Inula britannica, exhibits potent antitumor activity against several human cancer cell lines. However, the molecular mechanism of OODBL in the induction of anticancer activity is still unclear.
METHODS AND RESULTS:
In the present study, we demonstrated that OODBL induced the occurrence of apoptosis in human leukemic (HL-60) cells and cell arrest at the S phase. On the other hand, activation of caspase-8, -9, and -3, phosphorylation of Bcl-2 and Bid, and increased release of cytochrome c from mitochondria into cytosolic fraction were detected in OODBL-treated HL-60 cells. We further demonstrated that production of reactive oxygen species (ROS), activation of mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways may play an important role in OODBL-induced apoptosis.
CONCLUSIONS:
The results from the present study highlight the molecular mechanisms underlying OODBL-induced anticancer activity.
Immunopharmacol Immunotoxicol. 2017 Aug;39(4):173-179.
1,6-O,O-Diacetylbritannilactone suppresses activation of mast cell and airway hyper-responsiveness.[Pubmed:
28447503 ]
Mast cells play critical roles in allergic disorders such as atopic dermatitis and allergic asthma. The aim of this study was to investigate the anti-inflammatory and anti-asthmatic activities of 1,6-O,O-Diacetylbritannilactone (OODBL) isolated from Inula japonica Thunb. (I. japonica) in a murine asthma model and bone marrow-derived mast cells (BMMCs).
METHODS AND RESULTS:
In an ovalbumin-induced asthma model, OODBL administration attenuated the airway hyper-responsiveness induced by aerosolized methacholine and serum IgE level in asthmatic mice. In vitro system, we found that OODBL reduced leukotriene C4 production and degranulation through the suppression of cytosolic phospholipase A2 phosphorylation and phospholipase Cγ-mediated Ca2+ influx in IgE/antigen-stimulated BMMCs.
CONCLUSIONS:
Taken together, OODBL may have therapeutic potential in the treatment of allergic diseases such as asthma.