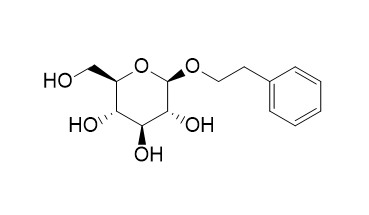
2-Phenylethyl-beta-glucopyranoside
Beta-glucosidase was highly specific for 2-phenylethyl beta-D-glucopyranoside.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Environ Toxicol.2020, doi: 10.1002
Front Nutr.2024, 11:1507886
Neurotox Res.2022, 40(6):1937-1947.
Molecules.2022, 27(19):6681.
Phytomedicine.2021, 84:153501.
BMC Plant Biol.2022, 22(1):128.
Nat Prod Commun.2017, 12(5):771-778
Cells.2022, 11(6):931.
Asian J Beauty Cosmetol2016, 14(3):249-257
Int J Nanomedicine.2022, 17:6513-6525.
Related and Featured Products
Phytochemistry . 2004 Apr;65(7):885-890.
ent-Kaurenoic acids from Mikania hirsutissima (Compositae)[Pubmed:
15081289]
Four ent-kaurenoic acid derivatives, 2beta,16alpha,17-trihydroxy-ent-kauran-19-oic acid (1), 3beta,16alpha,17-trihydroxy-ent-kauran-19-oic acid (2), 11alpha,15beta-dihydroxy-7-O-beta-d-glucopyranosyl-ent-kaur-16-en-19-oic acid (3) and 1alpha,15beta-dihydroxy-7-O-beta-d-glucopyranosyl-ent-kaur-16-en-19-oic acid (4), were isolated together with five known compounds, 1,5-dicaffeoyl-quinic acid (5), 2-O-glucosyloxy-4-methoxy-cinnamic acid (6), phenethyl alcohol glucoside (7), phenethyl-1-O-beta-d-apiofuranosyl (1-->2) beta-d-glucopyranoside (sayaendoside) (8) and 3,6-dihydroxy-beta-ion-9-ol (9) from the 50% aqueous acetone extract of the aerial parts of Mikania hirsutissima DC. (Compositae). Compounds 1-9 were tested for their proliferative activity toward peripheral blood mononuclear cells (hPBMC); compounds 1 and 2 showed significant activity (43.8% and 36.7%, at 100 microM, respectively) on the lymphocyte.
Biosci Biotechnol Biochem. 2008 Jan;72(1):219-221.
Purification and characterization of beta-glucosidase involved in the emission of 2-phenylethanol from rose flowers[Pubmed:
18175907]
Beta-glucosidase was partially purified from Rosa 'Hoh-Jun' petals. The enzyme was highly specific for such beta-D-glucopyranosides as 2-phenylethyl beta-D-glucopyranoside. The optimal activity was observed at pH 6.0 and 35 degrees C. The enzymes were composed with two proteins (160 and 155 kDa) by blue native-PAGE, and were classified in a family 1 glucosidase based on LC-MS/MS analyses.
Arch Pharm Res . 2008 Aug;31(8):983-988
Terpene and phenolic constituents of Lactuca indica L[Pubmed:
18787785]
We isolated seven terpenes and five phenolic constituents from the aerial parts of Lactuca indica L. using column chromatographic separation of its MeOH extract. Their structures were determined by spectroscopic methods to be trans-phytol (1), 3beta-hydroxyglutin-5-ene (2), 5,6-epoxy-3-hydroxy-7-megastigmen-9-one (3), 11beta-13-dihydrolactucin (4), 2-phenylethyl beta-D-glucopyranoside (5), cichorioside B (6), 1-hydroxylinaloyl-6-O-beta-D-glucopyranoside (7), (6S,9S)-roseoside (8), benzyl-beta-D-glucopyranoside (9), 2-(3'-O-beta-D-glucopyranosyl-4'-hydroxyphenyl)-ethanol (10), 3-(beta-D-glucopyranosyloxymethyl)-2-(4-hydroxy-3-methoxyphenyl)-5-(3-hydroxypropyl)-7-methoxy-dihydrobenzofuran (11), and (+)-taraxafolin-B (12). Compounds 1-3, 5, and 7-12 were isolated for the first time from this plant source. The isolated compounds were tested for cytotoxicity against four human tumor cell lines in vitro using a Sulforhodamin B bioassay.
Zhongguo Zhong Yao Za Zhi . 2016 Feb;41(4):689-693.
[Chemical structure and bioactivity of cis-5'-oxopropylnicotine][Pubmed:
28871694]
In this paper, the chemical constituents and bioactivities of leaves of Nicotiana tabacum were investigated. Six compounds were isolated by means of various chromatographic techniques (silica gel, Sephadex LH-20, MCI GEL CHP-20P and HPLC), and their structures were elucidated as cis-5'-(2-oxopropyl)-nicotine (1), 3-O-(9, 12, 15-octadecatrienoyl)-glyceryl-β-D-galactppyranoside (2), (l'R, 2R, 5S, 10R)-2-(1', 2'-dihydroxy-l'-methylethyl)-6, 10-dimethylspiro [4, 5] dec-6-en-8-one (3), (l'S, 2R, 5S, l0R)-2-(1', 2'-dihydroxy-l'-methylethyl)-6, 10-dimethylspiro [4, 5] dec-6-en-8-one (4),2, 3-dihydroxypropyl-β-D-galactoside (5) and phenylethyl β-D-glucopyranoside (6) by extensive spectroscopic analyses (UV, IR, MS, 1D- and 2D-NMR). Among them, compound 1 is a new alkaloid, and compounds 2-6 are isolated for the first time from N. tabacum. Compounds 1 and 2 were assayed for agitating activities on transient receptor potential vanilloid 1 (TRPV1), melatonin receptor 1 and 2 (MT1 and MT2), 1 showed agitating rate of 55.41% (1.53mmol•L⁻1) on MT2 and 2 possessed agitating rate of 128.11% (0.59 mmol•L⁻1) and 52.00% (0.73mmol•L⁻1) on TRPV1 and MT1, respectively.
Arch Pharm Res . 2008 Aug;31(8):983-988
Terpene and phenolic constituents of Lactuca indica L[Pubmed:
18787785]
We isolated seven terpenes and five phenolic constituents from the aerial parts of Lactuca indica L. using column chromatographic separation of its MeOH extract. Their structures were determined by spectroscopic methods to be trans-phytol (1), 3beta-hydroxyglutin-5-ene (2), 5,6-epoxy-3-hydroxy-7-megastigmen-9-one (3), 11beta-13-dihydrolactucin (4), 2-phenylethyl beta-D-glucopyranoside (5), cichorioside B (6), 1-hydroxylinaloyl-6-O-beta-D-glucopyranoside (7), (6S,9S)-roseoside (8), benzyl-beta-D-glucopyranoside (9), 2-(3'-O-beta-D-glucopyranosyl-4'-hydroxyphenyl)-ethanol (10), 3-(beta-D-glucopyranosyloxymethyl)-2-(4-hydroxy-3-methoxyphenyl)-5-(3-hydroxypropyl)-7-methoxy-dihydrobenzofuran (11), and (+)-taraxafolin-B (12). Compounds 1-3, 5, and 7-12 were isolated for the first time from this plant source. The isolated compounds were tested for cytotoxicity against four human tumor cell lines in vitro using a Sulforhodamin B bioassay.