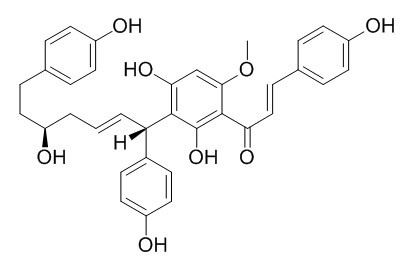
Calyxin B
Calyxin B exhibits potent activity against human HT-1080 fibrosarcoma cells with an ED50 value of 0.69 microM. A methylated product of calyxin A and an epimeric mixture of Calyxin B, showed greatly reduced activity suggesting that phenolic hydroxyl groups are involved in the inhibitory activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
AMB Express2020. 10(1):126.
Molecules 2022, 27(3),1047.
Phytomedicine.2018, 38:12-23
J Cell Physiol.2021, 236(3):1950-1966.
Biomed Chromatogr.2020, e5021.
J Agric Food Chem.2024, 72(40):22237-22249.
Phytochem Anal.2013, 24(5):493-503
J Pain Res.2022, 15:3469-3478.
J Drug Target.2016, 24:1-28
Food Chem.2024, 460(Pt 1):140472.
Related and Featured Products
Biol Pharm Bull. 1998 Apr;21(4):371-4.
Inhibitory effect of diarylheptanoids on nitric oxide production in activated murine macrophages.[Pubmed:
9586575]
METHODS AND RESULTS:
Thirteen novel diarylheptanoids bearing a chalcone or a flavanone moiety (1-13), a new curcumin derivative, 1,2-dihydrobis(de-O-methyl)curcumin (14), and two known flavonoids (15 and 16) isolated from the seeds of Alpinia blepharocalyx K. Schum. were tested for their inhibitory effects on nitric oxide (NO) production in lipopolysaccaride (LPS)-activated murine macrophages J774.1 in vitro. All the tested compounds inhibited NO production in a concentration-dependent manner (IC50=36-568 microM). Among the compounds examined, blepharoCalyxin B (13) was the most potent inhibitor of NO production (IC50=36 microM).
CONCLUSIONS:
Analysis of the structure activity relationship among these novel diarylheptanoids led to the conclusion that the position of attachment of a chalcone or a flavanone to a diarylheptanoid does not affect their inhibitory potency although their presence in association causes a substantial enhancement of the inhibitory activity. Moreover, a conjugated double bond in a chalcone moiety potentiated the inhibitory activity. On the other hand, hexamethoxydeoxycalyxin A (17) and pentamethoxyCalyxin B (18), a methylated product of calyxin A (1) and an epimeric mixture of Calyxin B, showed greatly reduced activity suggesting that phenolic hydroxyl groups are involved in the inhibitory activity.
Biol Pharm Bull. 2001 May;24(5):525-8.
Antiproliferative activity of diarylheptanoids from the seeds of Alpinia blepharocalyx.[Pubmed:
11379774]
METHODS AND RESULTS:
The 95% EtOH extract of the seeds of Alpinia blepharocalyx (Zingiberaceae) showed significant antiproliferative activity towards human HT-1080 fibrosarcoma and murine colon 26-L5 carcinoma cells.
Chemical investigation of the extract led to the isolation of forty-four new (1-44) and one known (45) diarylheptanoids, eleven phenolic compounds (46-56) together with beta-sitosterol glucoside (57). Almost all the isolated compounds showed significant antiproliferative activity in a concentration-dependent manner. Among the compounds, epicalyxin F (17) exhibited the most potent activity against the proliferation of colon 26-L5 carcinoma cells with an ED50 value of 0.89 microM, while Calyxin B (2) exhibited the most potent activity against human HT-1080 fibrosarcoma cells with an ED50 value of 0.69 microM. Moreover, calyxins B (2) and K (11), epicalyxins F (17), I (20) and K (22), 6-hydroxycalyxin F (25), blepharoCalyxin B (27) and mixtures of 7 and epicalyxin G (18) and of calyxin J (10) and epicalyxin J (21) possessed more potent activity than a clinically used anticancer drug, 5-fluorouracil, towards HT-1080 fibrosarcoma cells.
CONCLUSIONS:
Analysis of the structure activity relationship suggested that the position of the attachment of a chalcone or a flavanone moiety does not affect the activity, although their presence in association causes a substantial enhancement of the antiproliferative activity. Moreover, the conjugated double bond of the chalcone moiety and the phenolic hydroxyl group potentiate the antiproliferative activity of the compounds.