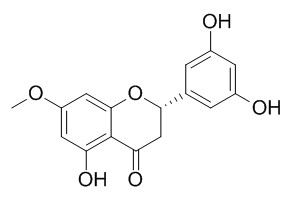
Blumeatin
Blumeatin has antioxidant properties, free radical scavenging activity,and has xanthine oxidase (XO) inhibitory activity. Blumeatin can protect liver against injury induced by CCl4 and TAA, it can inhibit the increase of serum alanine aminotransferase (AAT) and liver triglyceride and increased serum triglyceride, beta-lipoprotein, and liver glycogen content in CCl4-intoxicated rats, and can shorten the pentobarbital sleeping time in CCl4-intoxicated mice. Blumeatin can promote adipocyte differentiation as characterized by increased triglyceride levels in 3T3L1 cells, also can enhance the accumulation of lipid droplets and induced upregulation of the expression of the adipocyte-specific genes aP2 and GLUT4.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmacognosy Magazine2017, 13(52):868-874
Fundam. Toxicol. Sci.2024, 11(4):197-204
Advances in Traditional Medicine 2021, 21:779-789.
Int J Mol Sci.2019, 20(21):E5488
Pharmaceuticals (Basel).2024, ;17(8):1018.
Antiviral Res.2013, 98(3):386-93
Life Sci.2023, 332:122107.
Cancer Lett. 2023, 18:216584.
Toxicol In Vitro.2023, 93:105667.
Phytomedicine.2024, 128:155527.
Related and Featured Products
2',5,6',7-Tetrahydroxyflavanone
Catalog No: CFN97285
CAS No: 80604-16-6
Price: $318/10mg
2',5,6',7-Tetraacetoxyflavanone
Catalog No: CFN97286
CAS No: 80604-17-7
Price: Inquiry(manager@chemfaces.com)
Steppogenin
Catalog No: CFN98949
CAS No: 56486-94-3
Price: $413/5mg
3',5,5',7-Tetrahydroxyflavanone
Catalog No: CFN99673
CAS No: 160436-10-2
Price: Inquiry(manager@chemfaces.com)
Blumeatin
Catalog No: CFN99278
CAS No: 118024-26-3
Price: $368/10mg
Robtin
Catalog No: CFN98672
CAS No: 4382-34-7
Price: Inquiry(manager@chemfaces.com)
Plantagoside
Catalog No: CFN91894
CAS No: 78708-33-5
Price: Inquiry(manager@chemfaces.com)
3',4',5',5,7-Pentamethoxyflavanone
Catalog No: CFN95414
CAS No: 479672-30-5
Price: $218/20mg
5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavanone
Catalog No: CFN98393
CAS No: 310888-07-4
Price: Inquiry(manager@chemfaces.com)
5,6,7,3',4',5'-Hexamethoxyflavanone
Catalog No: CFN95376
CAS No: 74064-17-8
Price: $318/10mg
Food Chem., 2004, 88(2):243-52.
Free radical-scavenging activity of organic extracts and of pure flavonoids of Blumea balsamifera DC leaves.[Reference:
WebLink]
Phytochemical investigation on the leaves of Blumea balsamifera DC resulted in the isolation of 11 flavonoids. Their chemical structures were elucidated by means of elemental analyses and different spectroscopic methods, such as UV, IR, NMR and MS.
METHODS AND RESULTS:
The free radical scavenging activity of organic extracts of B. balsamifera DC leaves and that of pure flavonoids isolated from the leaves was evaluated using 1,1-diphenyl-2-picrylhydrazyl radical. A dose response curve was plotted for determining SC50 values (the concentrations required to inhibit radical formation by 50%). The antioxidant activities of crude extracts decreased in the order: methanol extract > chloroform extract > pet-ether extract. The antioxidant activities of all compounds tested decreased in the order: quercetin > rhamnetin > luteolin > luteolin-7-methylether > L-ascorbic acid > Blumeatin > butylated hydroxyanisole > 5,7,3',5'-tetrahydroxyflavanone > tamarixetin > butylated hydroxytoluene > alpha-tocopherol > dihydroquercetin-4'-methylether > dihydroquercetin-7,4'-dimethylether.
CONCLUSIONS:
This result indicates that flavonoid contents of different solvent extracts of B. balsamifera DC leaves were responsible for their antioxidant properties.
J Nat Prod. 2012 Apr 27;75(4):699-706.
Isoprenylated flavonoid and adipogenesis-promoting constituents of Dodonaea viscosa.[Pubmed:
22512738 ]
Ten new isoprenylated flavonol derivatives, dodoviscins A-J (1-10), and seven known compounds (11-17) were isolated from the aerial parts of Dodonaea viscosa.
METHODS AND RESULTS:
Compounds 1, 2, 4, 5, 7-9, 5,7,4'-trihydroxy-3',5'-bis(3-methyl-2-buten-1-yl)-3-methoxyflavone (11), 5,7,4'-trihydroxy-3',5'-bis(3-methyl-2-buten-1-yl)-3,6-dimethoxyflavone (12), 5,7,4'-trihydroxy-3'-(4-hydroxy-3-methylbutyl)-5'-(3-methyl-2-buten-1-yl)-3,6-dimethyoxyflavone (13), sakuranetin (14), and Blumeatin (15) promoted adipocyte differentiation as characterized by increased triglyceride levels in 3T3L1 cells. Compounds 1, 13, and 15 also enhanced the accumulation of lipid droplets and induced upregulation of the expression of the adipocyte-specific genes aP2 and GLUT4.
Zhongguo Yao Li Xue Bao. 1993 Jul;14(4):376-8.
Protective action of blumeatin against experimental liver injuries.[Pubmed:
8249641]
Blumeatin (Blu, 5,3',5'-trihydroxy-7-methoxy-dihydro-flavone) was first isolated from Blumea balsamifera DC by Department of Chemistry, Sunyatsen University of China.
METHODS AND RESULTS:
Blu ip inhibited the increase of serum alanine aminotransferase (AAT) and liver triglyceride and increased serum triglyceride, beta-lipoprotein, and liver glycogen content in CCl4-intoxicated rats. Histological lesions of liver were less severe than those of hepatic injury control. Blu ip 0.65 and 3.25 mg.kg-1 inhibited the increase of serum AAT and hepatic TG in thioacetamide (TAA)-intoxicated mice. Blu ip shortened the pentobarbital sleeping time in CCl4-intoxicated mice.
CONCLUSIONS:
It suggested that Blu could protect liver against injury induced by CCl4 and TAA.
Pharm Biol. 2010 Dec;48(12):1405-12.
Xanthine oxidase inhibitory activities of extracts and flavonoids of the leaves of Blumea balsamifera.[Pubmed:
20738223]
METHODS AND RESULTS:
The methanol extract (IC₅₀ = 0.111 mg/mL) showed higher XO inhibitory activity than the chloroform (0.138 mg/mL) and pet-ether extracts (0.516 mg/mL). IC₅₀ values of scavenging of superoxide radicals for extracts decreased in the order of: methanol (0.063 mg/mL) > chloroform (0.092 mg/mL) > pet-ether (0.321 mg/mL). The XO inhibitory activity of the isolated flavonoids and reference compounds tested decreased in the order of: allopurinol > luteolin > quercetin > tamarixetin > 5,7,3',5'-tetrahydroxyflavanone > rhamnetin > luteolin-7-methyl ether > Blumeatin > dihydroquercetin-4'-methyl ether > dihydroquercetin-7,4'-dimethyl ether > L-ascorbic acid.
CONCLUSIONS:
The results indicated that the flavone derivatives were more active than the flavonol derivatives. The flavanone derivatives were moderately active and the dihydroflavonol derivatives were the least. The higher flavonoid content of extracts contributed to their higher XO inhibitory activity.
Pak J Pharm Sci. 2013 Mar;26(2):375-81.
Simultaneous quantification of flavonoids in blood plasma by a high-performance liquid chromatography method after oral administration of Blumea balsamifera leaf extracts in rats.[Pubmed:
23455210]
The leaves of Blumea balsamifera are used as a folk medicine in kidney stone diseases in South-East Asia. Phytochemical investigation revealed leaves contained a number of flavonoids.
METHODS AND RESULTS:
In view of these, the present work was aimed to quantify and preliminary pharmacokinetic investigation of five flavonoids viz. dihydroquercetin-7,4¢-dimethyl ether (I), dihydroquercetin-4¢-methyl ether (II), 5,7,3¢,5¢-tetrahydroxyflavanone (III), Blumeatin (IV) and quercetin (V) in rat plasma following oral administration (0.5g/Kg) of B. balsamifera leaf extract in rats. Quantification was achieved by using a validated, reproducible high-performance liquid chromatographic method. The mean recoveries of dihydroquercetin-7,4¢-dimethyl ether (I), dihydroquercetin-4¢-methyl ether (II), 5,7,3¢,5¢-tetrahydroxyflavanone (III), Blumeatin (IV) and quercetin (V) were 90.6, 93.4, 93.5, 91.2 and 90.3% respectively. The limit of quantification was 25 ng/mL for I and IV, 10 ng/mL for II and III and 100 ng/mL for V respectively. The within day and day-to-day precision for all the compounds were < 10%. The validated HPLC method herein was applied for pharmacokinetic studies and the main pharmacokinetic parameters were: t1/2 (hr) 5.8, 4.3, 2.9, 5.7 and 7.3, Cmax (ng/mL) 594.9, 1542.9 1659.9, 208.9 and 3040.4; Tmax (hr) 4.7, 1.0, 1.0, 3.5 and 2.3; AUC0-oo (ng hr/mL) 5040, 5893, 9260, 1064 and 27233 for dihydroquercetin-7,4¢-dimethyl ether (I), dihydroquercetin-4¢-methyl ether (II), 5,7,3¢,5¢-tetrahydroxyflavanone (III), Blumeatin (IV) and quercetin (V) respectively.
CONCLUSIONS:
The developed method was suitable for pharmacokinetic studies and this preliminary study also revealed significant absorption after oral dosing in rats.