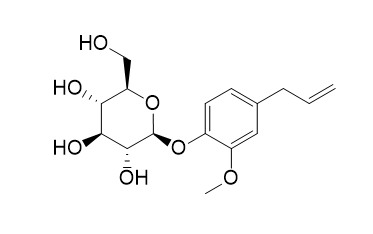
Citrusin C
citrusin c is a potential natural inhibitor against human serum albumin (SA)
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Srinagarind Medical Journal2017, 32(1)
J Ethnopharmacol.2020, 260:112988.
J Nat Med.2020, 74(1):65-75
Anticancer Res.2022, 42(9):4403-4410.
Plant Cell Physiol.2023, 64(7):716-728.
J Agric Food Chem.2024, 72(42):23183-23195
J Colloid Interface Sci.2022, 622:298-308.
J. Traditional Thai Medical Res. 2022,8(1):1-14.
Nat Commun.2024, 15(1):8221.
Fitoterapia.2022, 157:105130.
Related and Featured Products
Biomolecules . 2021 Jan 27;11(2):172.
Human Saliva-Mediated Hydrolysis of Eugenyl-β-D-Glucoside and Fluorescein-di-β-D-Glucoside in In Vivo and In Vitro Models[Pubmed:
33514072]
Eugenyl-β-D-glucopyranoside, also referred to as Citrusin C, is a natural glucoside found among others in cloves, basil and cinnamon plants. Eugenol in a form of free aglycone is used in perfumeries, flavourings, essential oils and in medicinal products. Synthetic Citrusin C was incubated with human saliva in several in vitro models together with substrate-specific enzyme and antibiotics (clindamycin, ciprofloxacin, amoxicillin trihydrate and potassium clavulanate). Citrusin C was detected using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Citrusin C was completely degraded only when incubated with substrate-specific A. niger glucosidase E.C 3.2.1.21 (control sample) and when incubated with human saliva (tested sample). The addition of antibiotics to the above-described experimental setting, stopped Citrusin C degradation, indicating microbiologic origin of hydrolysis observed. Our results demonstrate that Citrusin C is subjected to complete degradation by salivary/oral cavity microorganisms. Extrapolation of our results allows to state that in the human oral cavity, virtually all β-D-glucosides would follow this type of hydrolysis. Additionally, a new method was developed for an in vivo rapid test of glucosidase activity in the human mouth on the tongue using fluorescein-di-β-D-glucoside as substrate. The results presented in this study serve as a proof of concept for the hypothesis that microbial hydrolysis path of β-D-glucosides begins immediately in the human mouth and releases the aglycone directly into the gastrointestinal tract.
Curr Comput Aided Drug Des . 2020;16(3):308-317.
Identification of Novel Human Serum Albumin (SA) Inhibitors from Scoparia Dulsis for Urolithiasis[Pubmed:
31393255]
Background: Urolithiasis is the process of forming stones in the kidney, bladder, and/or urinary tract. It has been reported that kidney stones are the third most common disorder among urinary diseases. At present, surgical procedures and Extracorporeal Shock Wave Lithotripsy (ESWL) are commonly employed for the treatment of Urolithiasis. The major drawback of these procedures is the recurrence of stones.
Methods: This study aimed to identify potential natural inhibitors against human Serum Albumin (SA) from the plant Scoparia Dulsis for Urolithiasis. As protein-ligand interactions play a key role in structure- based drug design, this study screened 26 compounds from Scoparia Dulsis and investigated their binding affinity against SA by using molecular docking. The three dimensional (3D) structure of SA was retrieved from Protein Data Bank (PDB) and docked with PubChem structures of 26 compounds using PyRX docking tool through Autodock Vina. Moreover, a 3D similarity search on the PubChem database was performed to find the analogs of best scored compound and docking studies were performed. Drug-likeness studies were made using Swiss ADME and Lipinski's rule of five was performed for the compounds to evaluate their anti-urolithiatic activity.
Results: The results showed that Citrusin C (Eugenyl beta-D-glucopyranoside) exhibited best binding energy of -8.1 kcal/mol with SA followed by aphidicolin, apigenin, luteolin and scutellarein. Two compounds (PubChem CID 46186820, PubChem CID 21579141) analogous to Citrusin C were selected based on the lowest binding energy.
Conclusion: This study, therefore, reveals that these compounds could be promising candidates for further evaluation for Urolithiasis prevention or management.
Arch Pharm Res . 2004 Oct;27(10):1029-1033.
Phytochemical constituents ofCarpesium macrocephalum FR- et SAV[Pubmed:
27518388]
From the methanol extract of the whole plants ofCarpesium macrocephalum FR. et SAV., five sesquiterpene lactones (1: carabron,2: tomentosin,3: ivalin,4: 4H-tomentosin,5: carabrol) and three terpenoids (6: loliolide,7: vomifoliol,8: Citrusin C) were isolated. The structures and stereochemistry of compounds1-8 were established on the basis of chemical analysis as well as 1D- and 2D-NMR spectroscopy. Among them, compounds2, 4, and6-8 were isolated for the first time fromCarpesium species.
Magn Reson Chem . 2005 Jan;43(1):92-96.
NMR assignments and single-crystal X-ray diffraction analysis of deoxyloganic acid[Pubmed:
15505818]
7-Deoxyloganic acid (1), Citrusin C (2), 3,4-dihydroxyl benzoic acid (3) and (E)-caffeic acid (4) were isolated from the water-soluble fraction of ethanol extracts of Morina nepalensis var. alba Hand.-Mazz. and their structures were determined on the basis of spectroscopic evidence. The total assignments of 1H and 13C NMR spectra of 1 in solvents CD3OD, D2O and CDCl3 were reported, in addition to the single-crystal X-ray diffraction analysis of its tetraacetate 1a. All compounds were obtained from Morina genus for the first time.