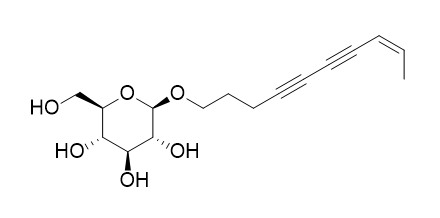
Bidenoside C
Bidenoside C may have hepatoprotective activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Evid Based Complement Alternat Med.2021, 2021:8707280.
Food Chem.2023, 424:136383.
Drug Test Anal.2018, 10(10):1579-1589
Sci Adv.2018, 4(10)
J Cell Physiol.2021, 236(3):1950-1966.
Chem Biol Interact.2024, 395:110999.
Molecules.2022, 27(4):1412.
Journal of Apiculture2023.38(3):249-254.
Int J Mol Sci.2023, 25(1):283.
World J.Traditional Chinese Med.2024, 10(3):370-382
Related and Featured Products
12-Hydroxyjasmonic acid
Catalog No: CFN99460
CAS No: 140631-27-2
Price: Inquiry(manager@chemfaces.com)
(S,E)-Deca-2,9-diene-4,6-diyne-1,8-diol
Catalog No: CFN97501
CAS No: 931114-98-6
Price: Inquiry(manager@chemfaces.com)
(R,E)-Deca-2-ene-4,6-diyne-1,8-diol
Catalog No: CFN97502
CAS No: 931116-24-4
Price: Inquiry(manager@chemfaces.com)
Panaxydol
Catalog No: CFN92797
CAS No: 72800-72-7
Price: $448/20mg
Panaxydiol
Catalog No: CFN92798
CAS No: 63910-76-9
Price: $368/10 mg
Panaxytriol
Catalog No: CFN95665
CAS No: 87005-03-6
Price: $368/10mg
Panaxyne
Catalog No: CFN96202
CAS No: 122855-49-6
Price: Inquiry(manager@chemfaces.com)
8-Acetoxypentadeca-1,9Z-diene-4,6-diyn-3-ol
Catalog No: CFN97743
CAS No: 41682-30-8
Price: Inquiry(manager@chemfaces.com)
9,17-Octadecadiene-12,14-diyne-1,11,16-triol
Catalog No: CFN98061
CAS No: 211238-60-7
Price: Inquiry(manager@chemfaces.com)
1-Acetoxy-9,17-octadecadiene-12,14-diyne-11,16-diol
Catalog No: CFN98068
CAS No: 213905-35-2
Price: Inquiry(manager@chemfaces.com)
Journal of Liquid Chromatography & Related Technologies, 2015, 38(19):1740-1746.
Pharmacokinetics of Five Different Types of Safflower Constituents in Rat Plasma after Oral Administration of Safflower Powder.[Reference:
WebLink]
The petals of Carthamus tinctorius L. (safflower) is an important traditional medicine for promoting blood circulation and removing blood stasis. The pharmacokinetic properties of multiple safflower constituents have not been fully understood.
METHODS AND RESULTS:
In the present research, an ultra-high-performance liquid chromatography-triple quadrupole mass method in dynamic multiple reaction monitoring mode was developed and validated to simultaneously quantify in rat plasma of five different characteristic types of safflower constituents, including chalcone, poly-acetylene, glycoside of 6-hydroxykaempferol, etc. All the five constituents, hydroxysafflor yellow A, Bidenoside C, 6-hydroxykaempferol-3,6-diglucoside, chlorogenic acid, and p-coumaric acid were detected in rat plasma after oral administration of safflower powder. p-Coumaric acid was the most quickly absorbed one with Tmax of 0.1 hr. The poly-acetylene constituent, Bidenoside C, displayed the largest AUC0-t value (2177.796 ng · h/L) followed by hydroxysafflor yellow A (1564.1 ng · h/L).
CONCLUSIONS:
The impressively favorable pharmacokinetic behavior of Bidenoside C reported herein for the first time rendered the importance for paying more attention to its role in the in vivo pharmacologic effects of safflower.
BMC Complement Altern Med, 2016 Jun 13;16:179.
Hepato-protective effects and chemical constituents of a bioactive fraction of the traditional compound medicine-Gurigumu-7.[Pubmed:
27296281]
Gurigumu-7 is an important traditional Mongolian medicine frequently used for liver diseases. However, the pharmacological effects and the bioactive constituents are not well understood.
METHODS AND RESULTS:
This research was to use CCl4-induced liver damage in mice to investigate the hepatoprotective effects of Gurigumu-7 and the methanol eluted fraction from a DIAION column of an extract of Gurigumu-7 (MF). The chemical constituents of MF were analyzed by UPLC-MS.
Pretreated orally with MF (66, 132 and 264 mg/kg) once a day for 4 days dose-dependently suppressed CCl4-induced mice liver histopathological changes and serum aminotransferase activities (alanine transaminase: 1144.0 ± 787.2 v.s. 2461.8 ± 1072.7 U/L, p < 0.05; aspartate transaminase: 1173 ± 785.3 v.s. 2506.6 ± 1140.7 U/L, p < 0.01). MF treated group demonstrated increased levels of SOD (108.19 ± 30.32 v.s. 75.75 ± 5.37 U/mg protein, p < 0.01) but decreased levels of malonyldialdehyde (7.68 ± 1.95 v.s. 44.32 ± 16.68 nmol/mg protein, p < 0.01) compared to the CCl4 control group. More than 30 chemical constituents were quantified, and MF was found to be rich in ellagic acid (297.97 mg/g), luteolin and its glucosides (35.10 mg/g), apigenin and its glucosides (>30 mg/g), ursolic acid (14.91 mg/g), Bidenoside C (8.75 mg/g), and proanthocyanidins (15.64 mg/g in proanthocyanidin A2 equivalent).
CONCLUSIONS:
The methanol eluted fraction (MF) from a DIAION column of an extract of the Mongolian medicine-Gurigumu-7 was found to be more hepatoprotective than Gurigumu-7. The results suggested that MF is a promising bioactive fraction for the development of new hepatoprotective medicine with better formulation and quality control properties.