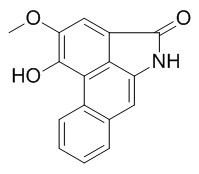
Aristolactam FI
Aristolactam FI shows platelet aggregation inhibitory activity.It is a potential cancer chemotherapeutic and chemopreventive agent.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Exp Biol Med (Maywood).2019, 244(18):1665-1679
Preprints2022, 2022030063.
Bull. Natl. Mus. Nat. Sci.2021, 47(2),109-114.
Analytical methods2019, 11(6)
Int J Mol Sci.2022, 23(23):14826.
Eur J Pharmacol.2024, 963:176280.
Food Sci Nutr.2023, 00:1-10.
Phytomedicine.2015, 22(14):1262-8
J Med Food.2022, 25(3):272-280.
Biochem Biophys Res Commun.2020, 522(4):1052-1058
Related and Featured Products
Bioorg Med Chem Lett. 2009 Jun 1;19(11):3036-40.
Synthesis of aristolactam analogues and evaluation of their antitumor activity.[Pubmed:
19394218]
A series of natural aristolactams and their analogues have been prepared and evaluated for antitumor activity against human cancer cells, including multi-drug resistant cell lines.
METHODS AND RESULTS:
Naturally occurring aristolactams, such as aristolactam BII (cepharanone B), aristolactam BIII, Aristolactam FI (piperolactam A), N-methyl piperolactam A, and sauristolactam showed moderate antitumor activities in selected cell lines.
However, several synthetic aristolactam derivatives exhibited potent antitumor activities against a broad array of cancer cell lines with GI(50) values in the submicromolar range.
Org Lett. 2008 Aug 21;10(16):3543-6.
Total synthesis of aristolactams via a one-pot suzuki-miyaura coupling/aldol condensation cascade reaction.[Pubmed:
18642834]
METHODS AND RESULTS:
A direct one-pot synthesis of phenanthrene lactams, which employs a Suzuki-Miyaura coupling/aldol condensation cascade reaction of isoindolin-1-one with 2-formylphenylboronic acid, has been developed. The approach is used to efficiently produce a number of natural aristolactams, such as aristolactam BII (cepharanone B), aristolactam BIII, Aristolactam FI (piperolactam A), N-methyl piperolactam A, and sauristolactam.