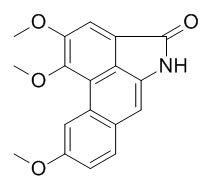
Aristolactam BIII
Aristolactam BIII possesses anti-platelet aggregation activity in vitro, it also shows significant cytotoxic activity (IC50 values < 4 microg/mL) against P-388, HT-29 and A549 cell lines in vitro.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biomolecules.2024, 14(10):1257.
Egyptian Pharmaceutical Journal2024, epj_205_23.
Cell Chem Biol.2019, 26(1):27-34
J Appl Biol Chem.2021, 64(3),263?268
Nutrients2020, 12(2):488
J Ethnopharmacol.2024, 326:117902.
J. ISSAAS2023, 29(2):36-51.
Mol Med Rep.2015, 12(5):7789-95
Front Pharmacol.2020, 11:566490.
Vietnam Journal of Science2022,64(2):69-75.
Related and Featured Products
Bioorg Med Chem Lett. 2009 Jun 1;19(11):3036-40.
Synthesis of aristolactam analogues and evaluation of their antitumor activity.[Pubmed:
19394218]
A series of natural aristolactams and their analogues have been prepared and evaluated for antitumor activity against human cancer cells, including multi-drug resistant cell lines.
METHODS AND RESULTS:
Naturally occurring aristolactams, such as aristolactam BII (cepharanone B), Aristolactam BIII, aristolactam FI (piperolactam A), N-methyl piperolactam A, and sauristolactam showed moderate antitumor activities in selected cell lines.
However, several synthetic aristolactam derivatives exhibited potent antitumor activities against a broad array of cancer cell lines with GI(50) values in the submicromolar range.
Planta Med. 2005 Jun;71(6):535-42.
New cytotoxic cyclobutanoid amides, a new furanoid lignan and anti-platelet aggregation constituents from Piper arborescens.[Pubmed:
15971125]
METHODS AND RESULTS:
Three new cyclobutanoid amides with trans-trans-trans configurations, piperarborenine C, piperarborenine D and piperarborenine E, and a new furanoid lignan, (+)-arborone, together with twelve known compounds, were isolated from the stems of Piper arborescens. The structures of these new compounds were determined by means of spectral analyses. Piperarborenine C, (+)-diayangambin, piplartine, piperolactam B, piperolactam C, Aristolactam BIII, goniothalactam, and methyl trans-3,4,5-trimethoxycinnamate possessed anti-platelet aggregation activity in vitro. Among them, piplartine showed the most potent anti-platelet aggregation activity induced by collagen and showed an IC50 value of 21.5 microM.
CONCLUSIONS:
Piperarborenines A - E, piperarborenine, aristololactam BIII and goniothalactam showed significant cytotoxic activity (IC50 values < 4 microg/mL) against P-388, HT-29 and A549 cell lines in vitro.