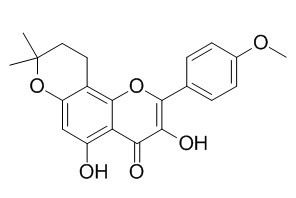
Anhydroicaritin
Anhydroicaritin exhibits immunosuppressive effects and has strong activity in scavenging DPPH radical. Anhydroicaritin can improve diet-induced obesity and hyperlipidemia and alleviate insulin resistance by suppressing SREBPs activation, it can serve as a leading compound for pharmacological control of metabolic diseases. Anhydroicaritin has the potential of stimulating the formation of mineralization nodules and further speeding up the formation of bone, it possesses significant protective effects on the zymosan-induced peritonitis mice, which may be associated with the regulation ofCa2+, influx in macrophages and iNOS expression.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2022, 27(13):4227.
Molecules.2021, 26(2):313.
Universidade Estadual Paulista2017, 42785
Biomed Pharmacother.2021, 144:112300.
Plant Physiol.2023, 193(3):1758-1771.
Cell Death Differ.2021, 1-8.
J Enzyme Inhib Med Chem.2019, 34(1):134-143
Chinese Pharmaceutical Journal2023, 58(2):178-187.
Phytomedicine.2021, 93:153796.
Front Pharmacol.2022, 13:919230.
Related and Featured Products
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012 Apr;28(4):374-6.
Effects of anhydroicaritin on the immunologic function of mouse macrophages.[Pubmed:
22482407]
To investigate the effects of Anhydroicaritin (AHI) on the immunologic function of mouse macrophages stimulated by lipopolysaccharide (LPS) in vitro and its related immunosuppressive mechanism.
METHODS AND RESULTS:
Mouse bone marrow-derived macrophages were isolated. Then, the drug toxicology of different concentrations of AHI on macrophages was measured by CCK-8 assay. The amount of NO produced in macrophages was detected by Griess kit. The phagocytosis of macrophages to E.coli BioParticles was assayed by flow cytometry (FCM). The expression of CD69, which was the marker of early activation of macrophages, was measured by FCM combined with two-color immunofluorescent staining of cell surface antigen. Cytometric bead array (CBA) kit was used to detect the production of cytokines of macrophages stimulated by LPS.
AHI (2.5, 5, 10 μmol/L) significantly reduced the production of NO in macrophages stimulated by LPS, and inhibited the phagocytosis of activated macrophages. The results of FCM analysis showed that AHI decreased proportions of CD69 on LPS-stimulated macrophages. Furthermore, AHI downregulated the secretion of cytokines of LPS-induced macrophages.
CONCLUSIONS:
AHI, which exhibits immunosuppressive effect on the mouse macrophages stimulated by LPS, is promising to be developed as an immunosuppressive reagent.
Journal of Shaanxi Normal University, 2012, 40(3):73-6.
Study on antioxidant activities of icariin and anhydroicaritin[Reference:
WebLink]
The antioxidant activities of the icariin and Anhydroicaritin were investigated by using 1,1-diphenyl-2-picryl-hydrazyl radical scavenging assay(DPPH·),superoxide radical scavenging assay(O2-·),hydroxyl radical scavenging assay(OH·),total antioxidant activity,and lipid peroxidation assay.
METHODS AND RESULTS:
The experiment results show that icariin exhibites strong activity in scaverging DPPH radical,superoxide radical and total antioxidation.Anhydroicaritin has stronger activity in scavenging DPPH radical than icariin,and effective inhibition against lipid peroxidation.The radical-scavenging and inhibition effects of icariin and Anhydroicaritin are dose-dependent.
CONCLUSIONS:
These results are useful for investigation and application of icariin and Anhydroicaritin.
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013 Oct;29(10):1036-9.
Protective effect of anhydroicaritin against peritonitis in mice.[Pubmed:
24103264 ]
To investigate the effect of Anhydroicaritin (AHI) against zymosan-induced peritonitis in mice.
METHODS AND RESULTS:
Peritonitis was induced in mice by intraperitoneal injection of zymosan. All mice were monitored for systemic toxicity and mortality for 13 d after zymosan or saline administration. In another set of experiments, the peritoneal exudates were collected. The leukocyte numbers and the production of inflammatory cytokines (IL-6, IL-10, TNF-α, MCP-1) were determined by flow cytometry. The release of nitric oxide (NO) was measured by a Griess reagent system. The Ca(2+); influx in bone marrow-derived macrophages was recorded by laser scanning confocal microscopy with Fluo4-AM loading. The expression of iNOS was determined by Western blotting.
AHI (4 mg/kg) prolonged survival of peritonitis mice, inhibited massive leukocyte transmigration into the peritoneal cavity, and decreased the overproduction of NO, IL-10, TNF-α, MCP-1 and IL-6. In LPS-stimulated mouse macrophages, AHI (5 μmol/L) pretreatment significantly inhibited the elevation of intracellular Ca(2+);, and markedly decreased iNOS protein expression.
CONCLUSIONS:
AHI possesses significant protective effects on the zymosan-induced peritonitis mice, which might be associated with the regulation of Ca(2+); influx in macrophages and iNOS expression.
Biochem Pharmacol. 2016 Dec 15;122:42-61.
Anhydroicaritin improves diet-induced obesity and hyperlipidemia and alleviates insulin resistance by suppressing SREBPs activation.[Pubmed:
27816546 ]
SREBPs play important roles in the regulation of lipid metabolism, and are closely related to the occurrence and development of many metabolic diseases.
Small molecular inhibitors of SERBPs are important tools in developing efficient treatment of metabolic diseases. However, there are no listing drug targeting SREBPs. Therefore, there is an urgent need to develop highly specific small molecules that inhibit SREBPs.
METHODS AND RESULTS:
In this study, using a hepatocyte-based high-throughput screening, we identified Anhydroicaritin (AHI) as a novel inhibitor of SREBPs. HepG2, HL-7702, and human primary hepatocytes were used to verify the effects of AHI. We explored the mechanism by which AHI blocks the binding of SCAP/SREBPs complex with Sec23α/24D via regulating LKB1/AMPK/mTOR pathway. AHI reduced liver cell lipid level by preventing de novo lipogenesis. In diet induced obese mice, AHI ameliorated obesity, insulin resistance, fatty accumulation in liver and hyperlipemia.
CONCLUSIONS:
In conclusion, AHI improves diet-induced obesity and alleviates insulin resistance by suppressing SREBPs maturation which is dependent on LKB1/AMPK/mTOR pathway. Thus, AHI can serve as a leading compound for pharmacological control of metabolic diseases.
Nat Prod Commun. 2012 Nov;7(11):1461-4.
Effects of anhydroicaritin and 2[Pubmed:
23285807]
Epimedium brevicornu Maxim., one of the most frequently used traditional Chinese medicines for thousands of years, is prescribed as having "bone strengthening" function and the ability to cure bone diseases.
METHODS AND RESULTS:
The present study evaluated the osteogenic effects of Anhydroicaritin (1) and 2"-hydroxy-3"-enAnhydroicaritin (2) isolated from E. brevicornu by activity-guided assay. Treatment with 1 and 2 improved the proliferation of murine osteoblastic MC3T3-E1 cells at doses of 10(-7)-10(-5) mol/L and 10(-7)-10(-6) mol/L, respectively, in the 72-hour culture period. Enzyme linked immunosorbent assay and histochemical staining demonstrated that both of these two prenyl-flavonoids significantly promoted the differentiation of MC3T3-E1 cells by enhancing the level of ALP activity in the cells.
CONCLUSIONS:
Alizarin Red staining and mineralized nodule quantification showed that 1 and 2 had the potential of stimulating the formation of mineralization nodules and further speeding up the formation of bone, indicating that both compounds might be potential candidates for bone regenerative medicine.