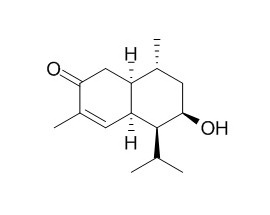
8-Hydroxy-4-cadinen-3-one
8-Hydroxy-4-cadinen-3-one shows significant inhibitory activity against A.thaliana seedling root growth.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2017, 19(1)
New Journal of Chemistry2019, 43:12538-12547
Industrial Crops and Products2024, 129:119014
Sci Rep. 2018, 462(8)
Molecules.2020, 25(23):5556.
Cells.2021, 10(11):2919.
J Korean Soc Food Sci Nutr2023, 52(12):1248-1255
J Adv Res.2019, 17:85-94
J Nat Med.2021, doi: 10.1007.
Int Immunopharmacol.2024, 143(Pt 2):113486.
Related and Featured Products
Journal of Biosafety, 2011, 20 (3) :207-12.
Isolation and identification of a potential allelochemical from the invasive plant Eupatorium adenophorum[Reference:
WebLink]
METHODS AND RESULTS:
The chemical structures of the purified compounds were identified by spectroscopy, including MS and NMR techniques. The allelopathic potential of sub-fractions of EtOAc extract and isolated pure compounds as well as two literature reported terpenoid allelochemicals, 8-Hydroxy-4-cadinen-3-one (2) and 4,7(11)-cadinadiene-3,8-dione (3), and signaling compound JA-Ile, were all evaluated by detecting their inhibitory activity against seeds germination and seedling root growth using Arabidopsis thaliana as test plant.
A bioactive compound was isolated from one polar sub-fraction of EtOAc extract of E.adenophorum and its chemical structure was identified as 2-coumaric acid glucoside (1). Bioassay indicated that compound 1 showed significant inhibitory activity against A.thaliana seedling root growth at concentration as low as 0.1 mmol·L-1, which is more potent than the other two identified compounds. A closely similar inhibition pattern of 1 to that of the signaling compound JA-Ile were also observed.
CONCLUSIONS:
In addition to less polar terpenoids, potentially existing large polar allelochemicals in E.adenophorum might significantly contribute the total allelopathic effects. The discovery of compound 1 and other potential polar allelochemicals from E.adenophorum may significantly promote the elucidation of the potential allelopathic mechanism possessed by this well-known invasive plant.