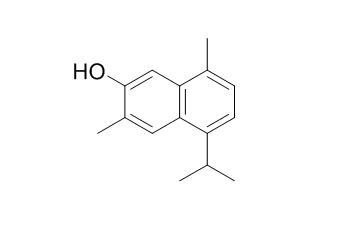
7-Hydroxycadalene
7-Hydroxycadalene shows cytotoxicity activity against HCT-15 cell line with IC50 18.89 ± 1.2 uM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Bioorg Chem.2024, 152:107720.
Antioxidants (Basel).2021, 10(11): 1802.
Jour. of Stored Pro & Postharvest Res.2016, 7(3):32-36
J Nat Med.2017, 71(2):457-462
J Ethnopharmacol.2024, 318:116863.
Industrial Crops and Products2018, 353-362
J Appl Biol Chem.2024, 67:33,238-244
University of Burgos2024, ssrn.4795441.
Faculty of Chem. & Nat. Resource Eng.2014, 62
LWT2020, 126:109313
Related and Featured Products
J Ethnopharmacol. 2015 Dec 4;175:256-65.
Toxicological evaluation of the natural products and some semisynthetic derivatives of Heterotheca inuloides Cass (Asteraceae).[Pubmed:
26344038 ]
Evaluate the potential toxicity of the natural products isolated from H. inuloides and some semisynthetic derivatives.
METHODS AND RESULTS:
The toxic aspects of the following natural products isolated from dried flowers of H. inuloides: 7-hydroxy-3,4-dihydrocadalene (1), 7-Hydroxycadalene (2), 3,7-dihydroxy-3(4H)-isocadalen-4-one (3), (1R,4R)-1-hydroxy-4H-1,2,3,4- tetrahydrocadalen-15-oic acid (4), D-chiro-inositol (5), quercetin (6), quercetin-3,7,3'-trimethyl ether (7), quercetin-3,7,3',4'-tetramethyl ether (8), eriodictyol-7,4'-dimethyl ether (9), α-spinasterol (10), caryolan-1,9β-diol (11) and 7-(3,3-dimethylallyloxy)-coumarin (12) as well as the toxic aspects of the semisynthetic compounds 7-acetoxy-3,4-dihydrocadalene (13), 7-benzoxy-3,4-dihydrocadalene (14), 7-acetoxycadalene (15), 7-benzoxycadalene (16), quercetin pentaacetate (17), 7-hydroxycalamenene (18), 3,8-dimethyl-5-(1-methylethyl)-1,2-naphthoquinone (19), and 4-isopropyl-1,6-dimethylbenzo[c]oxepine-7,9-dione (20). Toxic activities of compounds were determined by sulforhodamine B (SRB) assay, Artemia salina assay, RAW264.7 macrophage cells. Additionally, the acute toxicity in mouse of compound 1, the major natural sesquiterpene isolated from the acetone extract, was evaluated.The best cytotoxicity activity was observed for mansonone C (19) on K562 cell line with IC50 1.45 ± 0.14 μM, for 7-Hydroxycadalene (2) on HCT-15 cell line with IC50 18.89 ± 1.2 μM, and for quercetin pentaacetate (17) on MCF-7 cell line with IC50 22.57 ± 2.4 μM. Sesquiterpenes mansonone C (19) and 7-hydroxy-3,4-dihydrocadalene (1) caused the strongest deleterious effects against A. salina with IC50 39.4 ± 1.07, and 45.47 ± 1.74 μM, respectively. The number of viable RAW 264.7 cells was reduced with sesquiterpenes 1 and 2 by more than 90%. In addition, the acute study of 1 revealed no lethal effects at 300 mg/kg body weight, however, a reduction in the body weight of mice, morphological changes in the tissues of the liver and kidney and toxic signs were observed at very high doses (2000 mg/kg).
CONCLUSIONS:
The results provided evidence for the cytotoxicity of Mexican arnica (H. inuloides) metabolites and may be correlated with one of the popular uses of this plant, in traditional Mexican medicine, as anticancer remedy.
Among the active compounds contained in the acetone extract, the cytotoxic activity is mainly ascribable to cadinene type sesquiterpenes. In addition, evidence of acute toxicity suggests that 7-hydroxy-3,4-dihydrocadalene (1) may lead to toxicity at very high doses.
Eur J Pharmacol. 2010 Dec 15;649(1-3):154-60.
Antinociceptive effect of 7-hydroxy-3,4-dihydrocadalin isolated from Heterotheca inuloides: role of peripheral 5-HT₁ serotonergic receptors.[Pubmed:
20863828 ]
The purpose of this study was to investigate the possible antinociceptive effect of Heterotheca inuloides in inflammatory pain and to identify the main compounds involved in this effect.
METHODS AND RESULTS:
Dose-response curves were obtained for hexane, dichlorometane, ethyl acetate and methanol extracts from Heterotheca inuloides inflorescences in the formalin test. Hexane extract was more potent and effective than other extracts. Bio-guided fractionation was performed to determine the main antinociceptive compounds of the plant. Gas chromatography-mass spectrometry technique demonstrated the composition of the most active fraction from hexane extract revealing the presence of caryophyllene oxide, cedrene, 7-hydroxy-3,4-dihydrocadalin, 7-Hydroxycadalene and a compound not identified. The isolated compounds were individually evaluated in the formalin test in a preliminary dose of 100 μg/paw and only 7-hydroxy-3,4-dihydrocadalin showed a significant antinociceptive effect. Dose-response curves were then obtained for 7-hydroxy-3,4-dihydrocadalin and diclofenac, a prototypical analgesic drug. Both drugs were equieffective and equipotent in the second phase of the formalin test, but 7-hydroxy-3,4-dihydrocadalin was more effective and potent in the first phase than diclofenac. In addition, 7-hydroxy-3,4-dihydrocadalin reduced carrageenan-induced mechanical hyperalgesia and inflammation in a dose-dependent manner. Finally, in mechanistic studies, the antinociceptive effect of 7-hydroxy-3,4-dihydrocadalin in the formalin test was prevented by methiothepin, WAY100635, SB224289 and BRL15572 but not by naltrexone.
CONCLUSIONS:
Results support the use of H. inuloides inflorescences as analgesic in the Mexican traditional medicine. Moreover, data indicate that 7-hydroxy-3,4-dihydrocadalin is partly responsible of this pharmacological activity, and suggest that 5-HT(1A), 5-HT(1B), and 5-HT(1D) serotonergic, but not opioid, receptors participate in the antinociceptive effect of this drug.