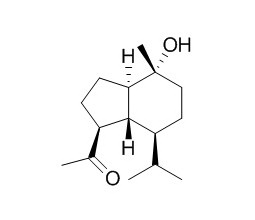
Oplopanone
Oplopanone exhibits noteworthy anti-plasmodial activity against Plasmodium falciparum strain.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Control Release.2021, 336:159-168.
Cardiovasc Toxicol.2021, 21(11):947-963.
Chin J Appl. Physiol.2019, 35(3):283-288
Food Chem Toxicol.2023, 176:113802.
Processes 2021, 9(5),894.
Int J Mol Sci.2023, 24(7):6360.
Curr Res Food Sci.2024, 9:100827.
Front Pharmacol.2021, 12:770667.
Environ Toxicol.2020, doi: 10.1002
J Ethnopharmacol.2017, 197:157-164
Related and Featured Products
Phytochemistry. 1999 Nov;52(6):1095-9.
Anti-plasmodial sesquiterpenoids from the African Reneilmia cincinnata.[Pubmed:
10643672]
METHODS AND RESULTS:
A new isodaucane sesquiterpenoid, 6,7,10-trihydoxyisodaucane, was isolated from the fruits of Reneilmia cincinnata, together with the known sesquiterpenoids oplodiol, Oplopanone, 5E,10(14)-germacradien-1 beta, 4 beta-diol, 1(10)E,5E-germacradien-4 alpha-ol and eudesman-1,4,7-triol. A large amount of 5-hydroxy-3,7,4'-trimethoxyflavone was also isolated. Their structures were established by NMR techniques using 1D and 2D experiments.
CONCLUSIONS:
Three of the known sesquirernenoids exhibited noteworthy anti-plasmodial activity against Plasmodium falciparum strains.
Nat Prod Res. 2012;26(9):850-8.
A new sesquiterpenoid from the rhizomes of Homalomena sagittifolia.[Pubmed:
21999629]
A new sesquiterpenoid, 1α,4β,7β-eudesmanetriol (1), was isolated together with the known compounds 1β,4β,7β-eudesmanetriol (2) and Oplopanone (3) from the rhizomes of Homalomena sagittifolia.
METHODS AND RESULTS:
The structures of these compounds were determined by extensive spectral analyses. The compounds 1 and 2 inhibited growth of Pseudomonas stutzeri with a MIC value of 117 µM when evaluated for antibacterial activity using the minimum concentration assay. Both these compounds showed remarkable activities against acetylcholinesterase enzyme with IC(50) values ranging between 25 and 26 µM.
CONCLUSIONS:
The isolation of these sesquiterpenoids and their biological activities observed in this study support the reported traditional uses of H. sagittifolia for the treatment of microbial related diseases and central nervous system disorders.
Arch Pharm Res. 2010 Sep;33(9):1317-23.
Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis ex vivo.[Pubmed:
20945129]
Five compounds previously known structures, scopoletin (1), northalifoline (2), stigmast-4-en-3-one (3), tiliroside (4), and Oplopanone (5) were obtained from the flower buds of Magnolia fargesii using chromatographic separation methods.
METHODS AND RESULTS:
The structures of 1-5 were identified by the interpretation of their spectroscopic data including 1D- and 2D-NMR as well as by comparison with reported values. Three compounds 1-3 were found from M. fargesii for the first time in this study. All the isolates (1-5) were subjected to in vitro bioassays to evaluate the inhibitory activity on advanced glycation end products formation and rat lens aldose reductase (RLAR). Compound 1 showed a remarkable inhibitory activity on advanced glycation end products formation with IC(50) value of 2.93 μM (aminoguanidine: 961 μM), and showed a significant RLAR inhibitory activity with IC(50) value of 22.5 μM (3.3-tetramethyleneglutaric acid: 28.7 μM). Compound 4 exhibited potent inhibitory activity against RLAR (IC(50) = 14.9 μM). In the further experiment ex vivo, cataractogenesis of rat lenses induced with xylose was significantly inhibited by compound 1 treatment.