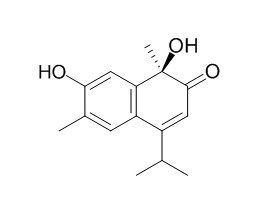
Lacinilene C
Lacinilene C, lacinilene C 7-methyl ether, scopoletin, 2-hydroxy-7-methoxycadalene and 2,7-dihydroxycadalene are 5 induced phytoalexins in cotton leaves. Lacinilene C methyl ether elicits contraction of TSM by enhancing the movement of calcium through potential-dependent channels of smooth muscle cell membranes.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Vietnam Journal of Science2022,64(2):69-75.
Phytother Res.2019, 33(4):1104-1113
Sci Rep.2017, 7:46299
TCI CO.2019, US20190151281A1
Plant Physiol.2024, 194(4):2580-2599.
LWT2020, 124:109163
Food Res Int.2022, 157:111397.
J Food Sci Technol.2022, 59(1):212-219.
Phytomedicine2022, 104:154318
Journal of Functional Foods2023, 104:105542
Related and Featured Products
Phytochemistry. 2015 Jul;115:59-69.
RNAi construct of a cytochrome P450 gene CYP82D109 blocks an early step in the biosynthesis of hemigossypolone and gossypol in transgenic cotton plants.[Pubmed:
25794893 ]
Naturally occurring terpenoid aldehydes from cotton, such as hemigossypol, gossypol, hemigossypolone, and the heliocides, are important components of disease and herbivory resistance in cotton.
METHODS AND RESULTS:
These terpenoids are predominantly found in the glands. Differential screening identified a cytochrome P450 cDNA clone (CYP82D109) from a Gossypium hirsutum cultivar that hybridized to mRNA from glanded cotton but not glandless cotton. Both the D genome cotton Gossypium raimondii and A genome cotton Gossypium arboreum possessed three additional paralogs of the gene. G. hirsutum was transformed with a RNAi construct specific to this gene family and eight transgenic plants were generated stemming from at least five independent transformation events. HPLC analysis showed that RNAi plants, when compared to wild-type Coker 312 (WT) plants, had a 90% reduction in hemigossypolone and heliocides levels, and a 70% reduction in gossypol levels in the terminal leaves, respectively. Analysis of volatile terpenes by GC-MS established presence of an additional terpene (MW: 218) from the RNAi leaf extracts. The (1)H and (13)C NMR spectroscopic analyses showed this compound was δ-cadinen-2-one.
CONCLUSIONS:
Double bond rearrangement of this compound gives 7-hydroxycalamenene, a Lacinilene C pathway intermediate. δ-Cadinen-2-one could be derived from δ-cadinene via a yet to be identified intermediate, δ-cadinen-2-ol. The RNAi construct of CYP82D109 blocks the synthesis of desoxyhemigossypol and increases the induction of Lacinilene C pathway, showing that these pathways are interconnected. Lacinilene C precursors are not constitutively expressed in cotton leaves, and blocking the gossypol pathway by the RNAi construct resulted in a greater induction of the Lacinilene C pathway compounds when challenged by pathogens.
Environ Res. 1988 Feb;45(1):118-26.
Lacinilene C methyl ether (LCME) constricts tracheal smooth muscle.[Pubmed:
3338430]
Lacinilene C methyl ether (LCME) is a constituent of the cotton plant and has been implicated as a causative agent of byssinosis.
METHODS AND RESULTS:
The effect of synthetic Lacinilene C methyl ether on the behavior of isolated strips of canine tracheal smooth muscle (TSM) was examined in tissue bath experiments. Lacinilene C methyl ether (0.64-6.6 x 10(-4) M) caused slowly developing but strong and sustained contractions of TSM strips. Blockade of acetycholine, 5-hydroxytryptamine (5-HT), and histamine H1 receptors with atropine, methysergide, and pyrilamine, respectively, had no effect on constrictions induced by Lacinilene C methyl ether. In contrast, the calcium channel antagonist, verapamil, significantly reduced the response to Lacinilene C methyl ether. Moreover, addition of the beta receptor agonist, isoproterenol, at the peak of Lacinilene C methyl ether-induced contractions relaxed tissues in a concentration-dependent manner.
CONCLUSIONS:
We conclude that Lacinilene C methyl ether elicits contraction of TSM by enhancing the movement of calcium through potential-dependent channels of smooth muscle cell membranes. This mechanism of action does not require activation of specific membrane receptors for acetylcholine, 5-HT, and histamine.
Phytochemistry, 1990 ,29 (6):1789-91.
Stress effects on cotton leaf phytoalexins elicited by cell free-mycelia extracts of Aspergillus flavus.[Reference:
WebLink]
METHODS AND RESULTS:
The concentrations of five induced phytoalexins, Lacinilene C, Lacinilene C 7-methyl ether, scopoletin, 2-hydroxy-7-methoxycadalene, and 2,7-dihydroxycadalene, were determined in cotton leaves treated with cell-free mycelial extracts of Aspergillus flavus under a light regime of 14 hr light/10 hr dark and temperature cycles of25/20°, 30/25°, 35/30° and 40/35°. Maximum concentrations were present in plants maintained at a post-inoculation temperature regime of 30/25°.
CONCLUSIONS:
In a separate experiment, the concentrations of the same elicited phytoalexins were measured in cotton leaves treated with the fungal elicitor and maintained at different levels of plant moisture stress. Leaf moisture stress values less than -11.7 bars resulted in decreased production of the five cotton leaf phytoalexins determined two days after the fungal elicitor was applied.