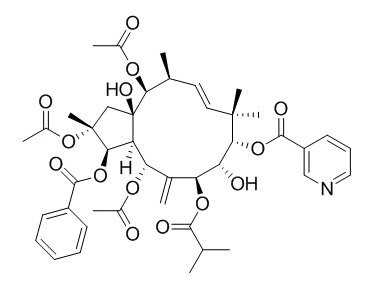
Jatrophane 3
(2R*,3R*,4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,9,14-Tetraacetoxy-3-benzoyloxy-8,15-dihydroxy-7-isobutyroyloxyjatropha-6(17),11E-diene(Jatrophane 4 ) and (2R*,3R*, 4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,14-triacetoxy-3-benzoyloxy-8,15-dihydroxy-7-isobutyroyloxy-9-nicotinoyloxyjatropha-6(17),11E-diene(Jatrophane 3) exhibit significant antifeedant activity against a generalist plant-feeding insect, the cotton bollworm (Helicoverpa armigera), with EC50 values of 0.36 and 0.43 ug/cm2, respectively.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chinese Pharmaceutical Journal2023, 58(2):178-187.
Front Pharmacol.2021, 12:615157.
Int Immunopharmacol. 2020, 83:106403.
Molecules 2021, 26(4),1092.
Front Plant Sci.2023, 14:1207940.
Plants (Basel).2022, 11(16):2126.
J Agric Food Chem.2022, 70(51):16176-16187.
J Ethnopharmacol.2017, 197:157-164
Front Cell Infect Microbiol.2018, 8:292
Plants (Basel).2021, 10(4):702.
Related and Featured Products
Phytochemistry. 2017 Apr;136:56-64
Chemical profile and defensive function of the latex of Euphorbia peplus.[Pubmed:
28062071 ]
Plant latex is an endogenous fluid secreted from highly specialized laticifer cells and has been suggested to act as a plant defense system. The chemical profile of the latex of Euphorbia peplus was investigated.
METHODS AND RESULTS:
A total of 13 terpenoids including two previously unknown diterpenoids, (2S*,3S*,4R*,5R*,6R*,8R*,l1R*,13S*,14S*,15R*, 16R*)-5,8,15-triacetoxy-3-benzoyloxy-11,16-dihydroxy-9-oxopepluane and (2R*,3R*, 4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,8,9,14-pentaacetoxy-3-benzoyloxy-15-hydroxy-7-isobutyroyloxyjatropha-6(17),11E-diene), ten known diterpenoids, and a known acyclic triterpene alcohol peplusol, were identified, using HPLC and UPLC-MS/MS analyses and through comparison with the authentic compounds isolated from the whole plant. The diterpenoids exhibited significant antifeedant activity against a generalist plant-feeding insect, the cotton bollworm (Helicoverpa armigera), with EC50 values ranging from 0.36 to 4.60 μg/cm2. In particular, (2R*,3R*,4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,9,14-tetraacetoxy-3-benzoyloxy-8,15-dihydroxy-7-isobutyroyloxyjatropha-6(17),11E-diene (Jatrophane 4)and (2R*,3R*, 4S*,5R*,7S*,8S*,9S*,l3S*,14S*,15R*)-2,5,14-triacetoxy-3-benzoyloxy-8,15-dihydroxy-7-isobutyroyloxy-9-nicotinoyloxyjatropha-6(17),11E-diene(Jatrophane 3) had EC50 values of 0.36 and 0.43 μg/cm2, respectively, which were approximately 7-fold more potent than commercial neem oil (EC50 = 2.62 μg/cm2). In addition, the major peplusol showed obvious antifungal activity against three strains of agricultural phytopathogenic fungi, Rhizoctonia solani, Colletotrichum litchi and Fusarium oxysporum f. sp. niveum.
CONCLUSIONS:
The results indicated that terpenoids in the latex of E. peplus are rich and highly diversified, and might function as constitutive defense metabolites against insect herbivores and pathogens for the plant.