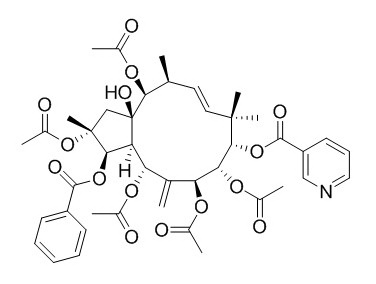
Pepluanin A
Pepluanin A, shows a very high activity for a jatrophane diterpene, outperforming cyclosporin A by a factor of at least 2 in the inhibition of Pgp-mediated daunomycin transport.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Colloid Interface Sci.2022, 622:298-308.
Molecules.2020, 25(23):5636.
Biochem Biophys Res Commun.2020, 527(4):889-895.
Auburn University2015, 1-58
J Pharm Biomed Anal.2017, 140:274-280
J Adv Res.2021, 35:245-257.
Asian Pac J Cancer Prev.2021, 22(S1):97-106.
Pharmaceuticals.2022, 15(4), 402.
Phytother Res.2022, 10.1002:ptr.7626.
Universitat Stuttgart2022, opus-12200.
Related and Featured Products
J Med Chem. 2004 Feb 12;47(4):988-92.
Jatrophane diterpenes as modulators of multidrug resistance. Advances of structure-activity relationships and discovery of the potent lead pepluanin A.[Pubmed:
14761200]
METHODS AND RESULTS:
From the whole plant of Euphorbia peplus L., five new diterpenes based on a jatrophane skeleton (pepluanins A-E, 1-5) were isolated, together with two known analogues (6 and 7), which served to divulge in detail the structure-activity relationships within this class of P-glycoprotein inhibitors. The results revealed the importance of substitutions on the medium-sized ring (carbons 8, 9, 14, and 15). In particular, the activity is collapsed by the presence of a free hydroxyl at C-8, while it increases with a carbonyl at C-14, an acetoxyl at C-9, and a free hydroxyl at C-15.
CONCLUSIONS:
The most potent compound of the series, Pepluanin A, showed a very high activity for a jatrophane diterpene, outperforming cyclosporin A by a factor of at least 2 in the inhibition of Pgp-mediated daunomycin transport.
Helvetica Chimica Acta , 2005 , 88 (6) :1560-1579.
Toward a Total Synthesis of Macrocyclic Jatrophane Diterpenes – Concise Route to a Highly Functionalized Cyclopentane Key Intermediate[Reference:
WebLink]
METHODS AND RESULTS:
A total synthesis of the biologically potent jatrophane diterpenes Pepluanin A (1) and euphosalicin A (2) is being aimed at. En route to these targets, a concise synthesis of the nonracemic cyclopentane building block 74 was developed. Key steps were a Claisen–Eschenmoser rearrangement of the enantiomerically enriched allylic alcohol 14 to amide 34 (Scheme 7), a hydroxy-lactonization of 40 to 43 (Scheme 9), followed by trans-lactonization to 72, which was subjected to a Davis hydroxylation to 69 (Scheme 17). Eventually, compound 69 was converted into the enol triflate 74.
CONCLUSIONS:
This material should prove suitable for an annulation of the macrocyclic ring characteristic of the desired jatrophanes 1 and 2. Less-successful approaches are also discussed due to their intrinsically valuable information content.