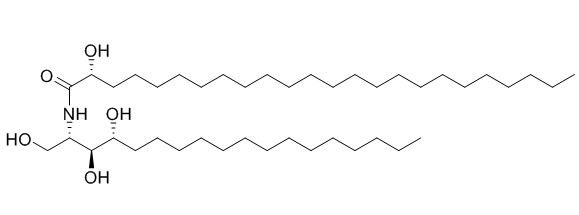
2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol
(2S,3S,4R,2′R)-2-(2′-hydroxytetracosanoylamino)octadecane-1,3,4-triol shows selectively inhibitory activity against phospholipase A 2 (PLA 2) secreted from Crotalus adamenteus venom at concentration of 100 ug/mL.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmaceuticals (Basel).2024, 18(1):19.
Antioxidants (Basel).2023, 12(12):2078.
Sci Rep.2018, 8(1)
Dermatologica Sinica2024, 42(1):p19-30.
Vietnam Journal of Science2022,64(2):69-75.
iScience.2023, 26(9):107602.
Pharmaceuticals (Basel).2024, ;17(8):1018.
GxABT2022, 2268.2:15515.
Biochem Biophys Res Commun.2019, 518(4):732-738
Biomed Pharmacother.2020, 125:109784.
Related and Featured Products
Chinese Journal of Organic Chemistry, 2003, 23(8): 853-7.
Chemical constituents of the fungus Leccinum extremiorientale.[Reference:
WebLink]
METHODS AND RESULTS:
Thirteen chemical constituents were isolated from the fruiting bodies of basidiomyctes Leccinum extremiorientale, and their structures were elucidated by spectral and physical-chemical data as: ergosta-7,22-dien3β,5α ,6β-triol (1), ergosterol peroxide (2), ergosterol (3), ergosta-4,6,8(14),22-tetraen-3-one (4), ergosta-5,7-dien-3β-ol (5), palmitic acid (6), (2S,3S,4R,2′R )-2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol (7), cerebroside B (8), cerebroside D (9), uracil (10), inosine (11), adeosine (12) and D-allitol (13). The NMR data of compounds 8 and 9 were completely assigned by 2D-NMR techniques, including 1H- 1H COSY, HMBC and HMQC.
CONCLUSIONS:
Compounds 2, 7 and 8 at concentration of 100 μg/mL showed selectively inhibitory activity against phospholipase A 2 (PLA2) secreted from Crotalus adamenteus venom, but inactive against PLA2 of bee venom (Apis mellifcra). In addition, the methanol-chloroform soluble extract of this fungus also showed 67% antifeedant rate against Leucaninia separata and caused 55% mortality against Plutella xylostella after three-day application.
Lipids. 2001 Feb;36(2):175-80.
A new ceramide from the basidiomycete Russula cyanoxantha.[Pubmed:
11269698]
METHODS AND RESULTS:
A new phytosphingosine-type ceramide (1) was isolated along with nine other compounds-5alpha,8alpha-epidioxy(22E,24R)-ergosta-6,22-dien-3beta-ol, 5alpha,8alpha-epidioxy-(24S)-ergosta-6-en-3beta-ol, (24S)-ergosta-7-ene-3beta,5alpha,6beta-triol, (22E,24R)-ergosta-7,22-dien-3beta,5alpha,6beta-triol, inosine, adenine, L-pyroglutamic acid, fumaric acid, and D-allitol from the ethanol and chloroform/methanol extract of the fruiting bodies of the basidiomycete Russula cyanoxanotha (Schaeff.) Fr.
CONCLUSIONS:
The structure of (1) was established as (2S,3S,4R,2'R)-2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol by means of spectroscopic and chemical methods.
Torosachrysone 8-O-beta-gentiobioside
Catalog No: CFN95131
CAS No: 94356-13-5
Price: $318/5mg
3,7,23,24-tetrahydroxycucurbita-5,25-dien-19-al
Catalog No: CFN95168
CAS No: 1446447-97-7
Price: $318/5mg
Clemomandshuricoside B
Catalog No: CFN95302
CAS No: 905294-48-6
Price: $318/10mg
Hederagenin 3-O-(2-O-acetyl-alpha-L-arabinopyranoside)
Catalog No: CFN95355
CAS No: 87562-05-8
Price: $318/10mg
4'-Hydroxy-3',5,5',6,7,8-hexamethoxyflavone
Catalog No: CFN95407
CAS No: 85644-03-7
Price: $318/5mg
Moreollic acid
Catalog No: CFN95437
CAS No: 173792-68-2
Price: $318/5mg
Ganoweberianic acid E
Catalog No: CFN95504
CAS No: 1309931-90-5
Price: $413/5mg
Caffeoylcalleryanin
Catalog No: CFN95532
CAS No: 20300-49-6
Price: $318/10mg
Mahuannin J
Catalog No: CFN95533
CAS No: N/A
Price: $318/5mg
8-Hydroxy-5,7-dimethoxyflavanone
Catalog No: CFN95580
CAS No: 201230-40-2
Price: $318/5mg