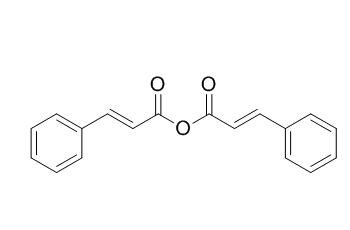
trans-Cinnamic anhydride
trans-Cinnamic anhydride is a acylating agent.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2020, 11:683.
Metabolites2023, 13(1), 3.
US20170000760 A12016, 42740
Molecules.2023, 28(7):3039.
Int. J. Mol. Sci.2022, 23(19), 11900.
Saudi Pharmaceutical Journal2023, 31(12):101829
The Journal of Korean Medicine2023, 44(4):26-40.
Separations2023, 10(4), 231.
Front Cell Infect Microbiol.2018, 8:292
Phytomedicine.2023, 116:154841.
Related and Featured Products
J Pharm Sci. 1983 Apr;72(4):369-72.
Solvent effects on the cinnamoylation of n-propyl alcohol catalyzed by N-methylimidazole and 4-dimethylaminopyridine.[Pubmed:
6864472]
METHODS AND RESULTS:
The kinetics of reaction of trans-Cinnamic anhydride or trans-cinnamoyl chloride with n-propyl alcohol, catalyzed by N-methylimidazole or 4-dimethylaminopyridine, were studied spectrophotometrically at 25 degrees in methyl ethyl ketone, ethylene dichloride, methylene chloride, and toluene. The acid chloride reacted in all solvents via the intermediate formation of the N-acyl catalyst, which underwent reaction with the alcohol catalyzed by another molecule of the base. The anhydride did not form the intermediate in any of the solvents, but underwent direct general base catalysis.
CONCLUSIONS:
The rate of the anhydride reactions was not sensitive to solvent polarity, whereas the rate of the chloride reactions tended to increase as the solvent polarity decreased.
A kinetic analysis is given of the effect of ion-pair formation on the kinetics of acyl transfer in systems where the charged N-acyl catalyst intermediate is formed.
J Pharm Sci. 1983 Apr;72(4):366-9.
Kinetics and mechanism of hydroxy compound cinnamoylation in acetonitrile catalyzed by N-methylimidazole and 4-dimethylaminopyridine.[Pubmed:
6864471]
METHODS AND RESULTS:
The kinetics of reaction of the acylating agents trans-Cinnamic anhydride and trans-cinnamoyl chloride with the hydroxy compounds n-propyl alcohol and water in the presence of N-methylimidazole and 4-dimethylaminopyridine were studied spectrophotometrically in acetonitrile solution at 25 degrees. The acid chloride reacted via the intermediate formation of the N-acyl catalyst, which underwent general base-catalyzed reaction with the hydroxy compound. The anhydride did not form the N-acyl intermediate, but instead underwent direct general base catalysis. In the presence of water, all systems formed the N-acyl intermediate.
CONCLUSIONS:
The mechanistic route followed by the system was determined by the nucleophilicity of the catalyst, the ability of the leaving group, and the polarity of the solvent.