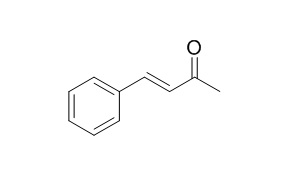
trans-4-phenylbut-3-en-2-one
trans-4-Phenylbut-3-en-2-one and 1,2-dichloro-4-nitrobenzene are marker substrates for the mouse Yb2 and Yb1 subunits respectively.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Structure2023, 36:100324.
Molecules.2023, 28(19):6767.
Front Pharmacol.2022, 13:919230.
The Pharmaceutical Society of Japan2018, 138(4):571-579
Biol Pharm Bull.2018, 41(1):65-72
LWT-Food Science and Technology2017, 75:488-496
Food Chem.2019, 275:746-753
Preprints2024, 2085.v1
Microbiol. Biotechnol. Lett.2022, 50(2): 193-201.
J Sep Sci.2020, 43(22):4148-4161.
Related and Featured Products
Biochem J. 1991 Jul 15;277 ( Pt 2):501-12.
Hepatic glutathione S-transferases in mice fed on a diet containing the anticarcinogenic antioxidant butylated hydroxyanisole. Isolation of mouse glutathione S-transferase heterodimers by gradient elution of the glutathione-Sepharose affinity matrix.[Pubmed:
1859377]
Induction of glutathione S-transferases (GSTs) is believed to represent an important mechanism whereby butylated hydroxyanisole inhibits chemical carcinogenesis.
METHODS AND RESULTS:
The soluble hepatic GSTs expressed by mice fed on normal diets are all homodimers comprising Ya3 (Mr 25,800), Yb1 (Mr 26,400) and Yf (Mr 24,800) subunits. In addition to these constitutively expressed GSTs, we have identified enzymes containing Ya1 (Mr 25,600), Ya2 (Mr 25,600), Yb2 (Mr 26,200) and Yb5 (Mr 26,500) subunits from the livers of Balb/c mice fed on diets containing butylated hydroxyanisole (BHA). Gradient affinity elution of GSH-Sepharose has been used to resolve the mouse liver enzymes into several discrete pools of activity from which GSTs were purified by cation-exchange chromatography.
CONCLUSIONS:
The inducible Mu-class Yb2 and Yb5 subunits were separately isolated as the heterodimers GST Yb1Yb2 and GST Yb1Yb5 and their catalytic properties are described; this showed that 1,2-dichloro-4-nitrobenzene and trans-4-phenylbut-3-en-2-one are marker substrates for the mouse Yb1 and Yb2 subunits respectively, but no discriminating model substrate was found that allows the identification of the Yb5 subunit.
Biochem J. 1985 Oct 15;231(2):263-7.
Kinetic independence of the subunits of cytosolic glutathione transferase from the rat.[Pubmed:
4062896 ]
The steady-state kinetics of the dimeric glutathione transferases deviate from Michaelis-Menten kinetics, but have hyperbolic binding isotherms for substrates and products of the enzymic reaction.
METHODS AND RESULTS:
The possibility of subunit interactions during catalysis as an explanation for the rate behaviour was investigated by use of rat isoenzymes composed of subunits 1, 2, 3 and 4, which have distinct substrate specificities. The kinetic parameter kcat./Km was determined with 1-chloro-2,4-dinitrobenzene, 4-hydroxyalk-2-enals, ethacrynic acid and trans-4-phenylbut-3-en-2-one as electrophilic substrates for six isoenzymes: rat glutathione transferases 1-1, 1-2, 2-2, 3-3, 3-4 and 4-4. It was found that the kcat./Km values for the heterodimeric transferases 1-2 and 3-4 could be predicted from the kcat./Km values of the corresponding homodimers. Likewise, the initial velocities determined with transferases 3-3, 3-4 and 4-4 at different degrees of saturation with glutathione and 1-chloro-2,4-dinitrobenzene demonstrated that the kinetic properties of the subunits are additive.
CONCLUSIONS:
These results show that the subunits of glutathione transferase are kinetically independent.