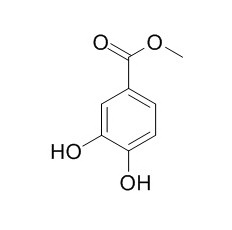
Protocatechuic acid methyl ester
Protocatechuic acid methyl ester has antiradical,and antimicrobial activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Ethnopharmacol.2020, 254:112733.
Kor. J. Herbol.2022, 37(5): 89-96.
AMB Express2020. 10(1):126.
FARMACIA2023, Vol.71,3.
Adv. Anim. Vet. Sci.2024, 12(4):732-741
Adv Healthc Mater.2024, 13(13):e2303276.
Tropical Journal of Pharmaceutical Research 2021, 20(6):1165-1170.
Korea Institute of Oriental Medicine2020, doi: 10.21203.
Bull.Natl.Mus.Nat.Sci.,Ser.B.2024, 50(2):79¨C86
Evid Based Complement Alternat Med.2022, 2022:1307173.
Related and Featured Products
Food Chem. 2014 Aug 1;156:312-8.
Degradation of cyanidin-3-rutinoside and formation of protocatechuic acid methyl ester in methanol solution by gamma irradiation.[Pubmed:
24629974]
Anthocyanins are naturally occurring phenolic compounds having broad biological activities including anti-mutagenesis and anti-carcinogenesis.
METHODS AND RESULTS:
We studied the effects and the degradation mechanisms of the most common type of anthocyanins, cyanidin-3-rutinoside (cya-3-rut), by using gamma ray. Cya-3-rut in methanol (1mg/ml) was exposed to gamma-rays from 1 to 10kGy. We found that the reddish colour of cya-3-rut in methanol disappeared gradually in a dose-dependent manner and effectively disappeared (>97%) at 10kGy of gamma ray. Concomitantly, a new phenolic compound was generated and identified as a Protocatechuic acid methyl ester by liquid chromatography, (1)H, and (13)C NMR.
CONCLUSIONS:
The formation of Protocatechuic acid methyl ester increased with increasing irradiation and the amount of Protocatechuic acid methyl ester formed by decomposition of cya-3-rut (20μg) at 10kGy of gamma ray was 1.95μg. In addition, the radical-scavenging activities were not affected by gamma irradiation.
J Nat Prod. 2014 Feb 28;77(2):424-8.
Sorbicatechols A and B, antiviral sorbicillinoids from the marine-derived fungus Penicillium chrysogenum PJX-17.[Pubmed:
24495078]
METHODS AND RESULTS:
Two novel sorbicillinoids combining a bicyclo[2.2.2]octane with a 2-methoxyphenol moiety, named sorbicatechols A (1) and B (2), were isolated from the culture of the marine sediment-derived fungus Penicillium chrysogenum PJX-17, together with the known Protocatechuic acid methyl ester and caffeic acid methyl ester (3). Their structures, including absolute configurations, were assigned by analysis of NMR, MS data, and TDDFT ECD calculations.
CONCLUSIONS:
Compounds 1 and 2 exhibited activities against influenza virus A (H1N1), with IC50 values of 85 and 113 μ M, respectively.
J Agric Food Chem. 2008 Jul 9;56(13):4928-36.
Reduction kinetics of the antiradical probe 2,2-diphenyl-1-picrylhydrazyl in methanol and acetonitrile by the antiradical activity of protocatechuic acid and protocatechuic acid methyl ester.[Pubmed:
18547047]
This work evaluates the reduction kinetics of the antiradical probe 2,2-diphenyl-1-picrylhydrazyl (DPPH (*)) in methanol and acetonitrile by the antiradical activity of protocatechuic acid (3,4-dihydroxybenzoic acid, 1) and Protocatechuic acid methyl ester ( 2).
METHODS AND RESULTS:
The reduction kinetics of DPPH (*) in both solvents by the antiradical activity of the p-catechol group in 2 is regular, that is, coincide with the proposed standard kinetic model for the reduction kinetics of DPPH (*) by the antiradical activity of an isolated p-catechol group. Therefore, the antiradical activity of 2 experimentally exhibits two rate-two stoichiometric constants in acetonitrile and three rate--three stoichiometric constants in methanol. In contrast, the reduction kinetics of DPPH (*) in both solvents by the antiradical activity of the p-catechol group in 1 is perturbed, that is, deviate from the proposed standard kinetic model.
CONCLUSIONS:
The deviations arise from the presence of the reactive carboxylic acid function which, in methanol, induces an additional reversible side reaction and, in acetonitrile, turns an irreversible reaction reversible, thus modifying the otherwise regular reduction kinetics of DPPH (*) by the antiradical activity of the p-catechol group in 1. On the other hand, the approximated theoretical kinetic equation that applies for those p-catechol groups whose reduction kinetics is regular and that experimentally exhibit three rate--three stoichiometric constants has been derived and used for fitting.