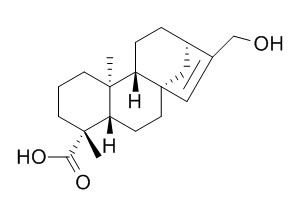
ent-17-Hydroxykaur-15-en-19-oic acid
ent-17-Hydroxykaur-15-en-19-oic acid shows cytotoxicity against human prostate (22Rv1, LNCaP), colon (HT29, HCT116, SW480, SW620), and breast (MCF-7) tumor cells at concentrations ranging from 6 to 50microg/mL.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Spectrochim Acta A2019, 210:372-380
Food Res Int.2018, 106:909-919
Biomed Pharmacother.2020, 128:110318.
Molecules.2019, 24(1):E159
Industrial Crops and Products2018, 353-362
Integr Cancer Ther.2018, 17(3):832-843
Biofactors.2018, 44(2):168-179
Konkuk University2023, 29:4634721
Neuroscience.2024, 559:77-90.
The Journal of Korean Medicine2023, 44(4):26-40.
Related and Featured Products
Cancer Lett. 2006 Dec 8;244(2):190-4.
Kaurene diterpenes from Laetia thamnia inhibit the growth of human cancer cells in vitro.[Pubmed:
16448743 ]
METHODS AND RESULTS:
Four ent-kaurene diterpenes were isolated from the leaves of Laetia thamnia L.: ent-kaur-16-en-19-oic acid (1a), ent-3beta-hydroxykaur-16-ene (2), ent-kaur-16-en-3alpha,19-diol (3a), and ent-17-Hydroxykaur-15-en-19-oic acid (4). The methyl ester (1b) of compound 1a and the acetate diester (3b) of compound 3a were prepared, and all compounds were evaluated for cytotoxicity against human prostate (22Rv1, LNCaP), colon (HT29, HCT116, SW480, SW620), and breast (MCF-7) tumor cells at concentrations ranging from 6 to 50microg/mL.
CONCLUSIONS:
The kaurenes showed activity in all cell lines tested, with the prostate cells demonstrating the most sensitivity as follows: 22 Rv1 cells towards 1a (IC(50) 5.03microg/mL) and 1b (IC(50) 6.81microg/mL), and LNCaP towards 2 (IC(50) 12.83microg/mL) and 4 (IC(50) 17.63microg/mL).
Ultrason Sonochem. 2014 Jul;21(4):1578-84.
Bio-guided optimization of the ultrasound-assisted extraction of compounds from Annona glabra L. leaves using the etiolated wheat coleoptile bioassay.[Pubmed:
24556321 ]
A bio-guided optimization of the extraction of bioactive components from Annona glabra leaves has been developed using the etiolated wheat coleoptile bioassay as the control method.
METHODS AND RESULTS:
The optimization of an ultrasound-assisted extraction of bioactive compounds using allelopathy results as target values has been carried out for the first time. A two-level fractional factorial experimental design was applied to optimize the ultrasound-assisted extraction. The solvent was the extraction variable that had the most marked effect on the resulting bioactivity of the extracts in the etiolated wheat coleoptile bioassay. Extraction time, extraction temperature and the size of the ultrasonic probe also influenced the bioactivity of the extracts. A larger scale extraction was carried out in the next step in the allelopathic study, i.e., the isolation of compounds from the bioactive extract and chemical characterization by spectroscopic techniques, including NMR. Eight compounds were isolated and identified from the active extracts, namely two steroids (β-sistosterol and stigmasterol), five diterpenes with the kaurane skeleton (ent-kaur-16-en-19-oic acid, ent-19-methoxy-19-oxokauran-17-oic acid, annoglabasin B, ent-17-Hydroxykaur-15-en-19-oic acid and ent-15β,16β-epoxy-17-hydroxy-kauran-19-oic acid) and the acetogenin asimicin.
CONCLUSIONS:
The most active compound was annoglabasin B, which showed inhibition with values of -95% at 10(-3) M, -87% at 5×10(-4) M and greater than -70% at 10(-4) M in the etiolated wheat coleoptile bioassay.
Phytochemistry.1993 Dec;34(6):1483–1487.
Occurrence and origin of ent-17-hydroxykaur-15-ene and ent-17-hydroxykaur-15-en-19-oic acid in shoots of Zea mays.[Reference:
WebLink]
Previously unidentified metabolites of ent-kaur-16-ene and ent-kaur-16-en-19-oic acid in shoots of maize are identified, respectively, as ent-17-hydroxykaur-15-ene and ent-17-Hydroxykaur-15-en-19-oic acid by GC-MS comparison with authentic samples.
METHODS AND RESULTS:
The production of the two latter compounds from the first two substances is analogous to the metabolism of gibberellin A5 to gibberellin A3 and provides two further examples, in plants, of hydroxylation at a double bond with rearrangement of the double bond.