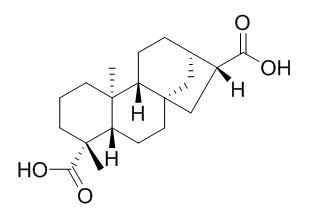
ent-Kauran-17,19-dioic acid
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Applied Biological Chemistry2022, 65(12)
South African J of Plant&Soil2018, 29-32
Food Science and Preservation2024, 31(3):486-498.
BMC Biotechnol.2024, 24(1):94.
Life (Basel).2021, 11(12):1399.
Aquaculture2019, 510:392-399
Bioorg Chem.2024, 145:107182.
bioRxiv - Biochemistry2023, 548213.
ACS Food Sci. Technol.2023, 3(2):273-282.
Nutrients2023, 15(18), 4016.
Related and Featured Products
Journal of Separation Science, 2012, 35(19).
Simultaneous determination of seven major diterpenoids in Siegesbeckia pubescensMakino by high‐performance liquid chromatography coupled with evaporative light scattering detection[Reference:
WebLink]
METHODS AND RESULTS:
A novel HPLC method with evaporative light scattering detection was developed for the simultaneous quantification of seven major diterpenoids of two types, including ent‐pimarane type: Kirenol, Hythiemoside B, Darutigenol, and ent‐kaurane type: ent‐16β,17,18‐trihydroxy‐kauran‐19‐oic acid, ent‐17,18‐dihydroxy‐kauran‐19‐oic acid, ent‐16β,17‐dihydroxy‐kauran‐19‐oic acid, 16α‐hydro‐ent-Kauran-17,19-dioic acid in the aerial parts of Siegesbeckia pubescens Makino, an important traditional Chinese medicinal herb. Chromatographic separation was achieved on a Waters Symmetry ShieldTM RP18 column (250 mm× 4.6 mm id, 5 μm) with a gradient mobile phase (A: 0.3% v/v aqueous formic acid and B: acetonitrile) at a flow rate of 1.0 mL/min. The drift tube temperature of evaporative light scattering detection was set at 103°C, and nitrogen flow rate was 3.0 L/min. The method was validated for accuracy, precision, LOD, and LOQ. All calibration curves showed a good linear relationship (r > 0.999) in test range. Precision was evaluated by intra‐ and interday tests that showed RSDs were less than 3.5%. Accuracy validation showed that the recovery was between 96.5 and 102.0% with RSDs below 2.8%.
CONCLUSIONS:
The validated method was successfully applied to determine the contents of seven diterpenoids in the different parts of Siegesbeckia pubescens Makino from two sources and to determine the contents of ent‐pimarane, ent‐kaurane, and total diterpenoids.