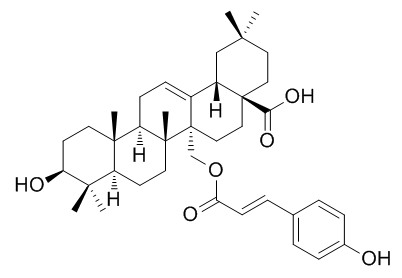
Uncarinic acid E
Uncarinic acid E has anti-cancer activity, it induces apoptosis in HepG2 cells via accumulation of p53, alters the Bax/Bcl-2 ratio, and activates caspases, resulting in cytochrome c release from the mitochondria. A mixture of uncarinic acid E and 27-O-p-(E)-coumaroyloxyursolic acid exhibits antiplasmodial activity, with the IC50 value of 2.9 microg/ml.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chemistry of Plant Materials.2016, 33-46
Molecules2022, 27(14),4462
Saudi Pharmaceutical Journal2023, 31(12):101829
Molecules.2020, 25(3):734
Plants2022, 11(3),294.
Molecules.2021, 26(2):E255.
LWT2021, 150:112021.
Front Pharmacol.2021, 12:615157.
Molecules.2024, 29(6):1240.
Fitoterapia.2021, 153:104995.
Related and Featured Products
J Nat Prod. 2000 Jun;63(6):753-6.
Inhibition of phospholipase cgamma1 and cancer cell proliferation by triterpene esters from Uncaria rhynchophylla.[Pubmed:
10869194]
Investigation of the hooks of Uncaria rhynchophylla resulted in isolation of six phospholipase Cgamma1 (PLCgamma1) inhibitors (1-6).
METHODS AND RESULTS:
The structures of these compounds were elucidated as pentacyclic triterpene esters by spectroscopic and chemical analysis. Three of them, namely uncarinic acid C (1), uncarinic acid D (2), and Uncarinic acid E (3), are newly reported as natural products.
CONCLUSIONS:
All the compounds showed dose-dependent inhibitory activities against PLCgamma1 in vitro with IC(50) values of 9.5-44.6 microM and inhibited the proliferation of human cancer cells with IC(50) values of 0.5-6.5 microg/mL.
J Ethnopharmacol. 2003 Oct;88(2-3):275-7.
Antiplasmodial triterpenes from twigs of Gardenia saxatilis.[Pubmed:
12963155]
METHODS AND RESULTS:
Ten triterpenes (1-10) were isolated and identified from the twigs of Gardenia saxatilis (Rubiaceae) and were subjected to antiplasmodial evaluation against the parasite Plasmodium falciparum. The first six compounds, lupenone (1), lupeol (2), betulinic acid (3), oleanolic acid (4), ursolic acid (5), and winchic acid (27-O-feruloyloxybetulinic acid) (6) were inactive in the assay. The other four compounds, messagenic acid A (7) and messagenic acid B (8), the 27-O-p-(Z)- and 27-O-p-(E)-coumarate esters of betulinic acid, and a mixture of Uncarinic acid E (27-O-p-(E)-coumaroyloxyoleanolic acid) (9) and 27-O-p-(E)-coumaroyloxyursolic acid (10) exhibited antiplasmodial activity, with the IC50 values of 1.5, 3.8 and 2.9 microg/ml, respectively.
CONCLUSIONS:
The results indicated that the p-coumarate moieties at the 27-position, both the cis and trans isomers, contributed to antiplasmodial activity. Introduction of a methoxyl group to the 3-position of the p-coumarate moiety to give a ferulate moiety resulted in loss of activity.
Biol Pharm Bull. 2006 Aug;29(8):1639-44.
The course of uncarinic acid E-induced apoptosis of HepG2 cells from damage to DNA and p53 activation to mitochondrial release of cytochrome c.[Pubmed:
16880619]
Uncarinic acid E, an active component isolated from Gelsemium elegans BENTH, has been reported to exhibit antitumor effects, but little is known about its molecular mechanisms of action.
METHODS AND RESULTS:
In this study, the growth-inhibitory activity of Uncarinic acid E for HepG2 cells is in time- and dose-dependent manner. HepG2 cells treated with Uncarinic acid E exhibited several typical characteristics of apoptosis through photomicroscopical observation, DNA agarose gel electrophoresis. The inhibitory effect of Uncarinic acid E on HepG2 cells was partially reversed by the inhibitors of pan-caspase, caspase-3 and caspase-6. The protein expression ratio of Bcl-xL/Bax and Bcl-2/Bax was down-regulated and Uncarinic acid E-induced apoptosis involves the initial phase mediated by the balance among Bcl-xL, Bcl-2 and Bax proteins, resulting in cytochrome c release from the mitochondria. Uncarinic acid E significantly increased the expression of p53 proteins indicates that p53 plays a pivotal role in the initiation phase of Uncarinic acid E-induced HepG2 cell apoptosis. The phoshatidylinositol 3-kinase (PI3-K) family inhibitor wortmanin and the MEK inhibitor (PD98059) rescued the viability loss induced by Uncarinic acid E through the expression of p53.
CONCLUSIONS:
Taken together, Uncarinic acid E induces apoptosis in HepG2 cells via accumulation of p53, alters the Bax/Bcl-2 ratio, and activates caspases, resulting in cytochrome c release from the mitochondria.
Chem Biodivers. 2017 Apr;14(4).
Cytotoxic Triterpenoids from the Barks of Betula platyphylla var. japonica.[Pubmed:
28052515 ]
METHODS AND RESULTS:
Phytochemical investigation on the barks of Betula platyphylla var. japonica (Betulaceae) was carried out, resulting in the isolation and identification of three new triterpenoids, 27-O-cis-caffeoylcylicodiscic acid (1), 27-O-cis-feruloylcylicodiscic acid (2), and 27-O-cis-caffeoylmyricerol (3), along with six known triterpenoids, obtusilinin (4), winchic acid (5), 27-O-trans-caffeoylcylicodiscic acid (6), Uncarinic acid E (7), myriceric acid B (8), and 3-O-trans-caffeoyloleanolic acid (9). The structures of the new compounds were elucidated by extensive spectroscopic methods, including 1D- and 2D-NMR, and HR-ESI-MS. All of the isolated compounds were evaluated for cytotoxicity against four human tumor cell lines (A549, SK-OV-3, SK-MEL-2, and Bt549).
CONCLUSIONS:
Compounds 2, 6, 8, and 9 exhibited potent cytotoxicity against all of the tumor cells tested (IC50 < 10.0 μm), while compounds 3, 4, 5, and 7 showed moderate cytotoxicity against all of the tumor cells tested (IC50 < 20.0 μm).