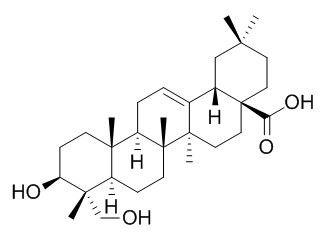
Hederagenin
Hederagenin shows anti-cancer,anti-inflammatory, antidepressant-like ,anticomplementary, and antimutagenic effects, it can evoke hemolysis on the erythrocytes, and has cytotoxic on various tumor cell lines, P-388, L-1210, U-937, HL-60, SNU-5 and HepG2. Hederagenin can inhibit LPS-stimulated expression of iNOS, COX-2, and NF-κB, regulate monoamine neurotransmitters and 5-HTT mRNA expression.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Bone Miner Res.2017, 32(12):2415-2430
J Agric Food Chem.2017, 65(13):2670-2676
J Food Compos Anal2017, 62:197-204
Cells.2022, 11(6):931.
Elife.2021, 10:e68058.
Korean J of Food Science&Technology 2017, 49(2):146-150
J Appl Biol Chem2022, 65:343−348.
Int J Mol Sci.2023, 24(8):7045.
J Agric Food Chem.2018, 66(1):351-358
Molecules.2021, 26(3):695.
Related and Featured Products
BMC Complement Altern Med. 2014 Oct 24;14:412.
Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway.[Pubmed:
25342273]
Colorectal cancer has become one of the leading cause of cancer morbidity and mortality throughout world. Hederagenin, a derivative of oleanolic acid isolated from the leaves of ivy (Hedera helix L.), has been shown to have potential anti-tumor activity. The study was conducted to evaluate whether Hederagenin could induce apoptosis of human colon cancer LoVo cells and explore the possible mechanism.
METHODS AND RESULTS:
MTT assay was used for evaluating cell viability while Annexin V-FITC/PI assay and Hoechst 33342 nuclear stainining were used for the determination of apoptosis and mitochondrial membrane potential. DCFH-DA fluorescence staining and flow cytometry were used to measure ROS generation. Real-time PCR and western blot analysis were performed for apoptosis-related protein expressions.
MTT assay showed that Hederagenin could significantly inhibit the viability of LoVo cells in a concentration-dependent and time-dependent manner by IC50 of 1.39 μM at 24 h and 1.17 μM at 48 h. The apoptosis ratio was significantly increased to 32.46% and 81.78% by the induction of Hederagenin (1 and 2 μM) in Annexin V-FITC/PI assay. Hederagenin could also induce the nuclear changes characteristic of apoptosis by Hoechst 33342 nuclear stainining under fluorescence microscopy. DCFH-DA fluorescence staining and flow cytometry showed that Hederagenin could increase significantly ROS generation in LoVo cells. Real-time PCR showed that Hederagenin induced the up-regulation of Bax and down-regulation of Bcl-2, Bcl-xL and Survivin. Western blotting analysis showed that Hederagenin decreased the expressions of apoptosis-associated proteins Bcl-2, procaspase-9, procaspase-3, and polyADP- ribosepolymerase (PARP) were increased, while the expressions of Bax, caspase-3, caspase-9 were increased. However, there was no significant change on caspase-8.
CONCLUSIONS:
These results indicated that the disruption of mitochondrial membrane potential might contribute to the apoptosis of Hederagenin in LoVo cells. Our findings suggested that Hederagenin might be a promising therapeutic candidate for human colon cancer.
Arch Pharm Res. 1999 Jun;22(3):317-9.
In vitro anticomplementary activity of hederagenin saponins isolated from roots of Dipsacus asper.[Pubmed:
10403139]
Anticomplementary activity of Hederagenin and related saponins isolated from Dipsacus asper was investigated in vitro.
METHODS AND RESULTS:
HN saponin F (3) was most potent with IC50 value of 3.7x10(-5) M followed by 3-O-beta-D-glucopyranosyl-(1->3)-alpha-L-rhamnopyranosyl-(1->2)-beta-L-+ ++arabi nopyranosyl Hederagenin 28-O-beta-D-glucopyranosyl-(1->6)-beta-D-glucopyrano side (8), 3-O-beta-L-arabinopyranosyl Hederagenin 28-O-beta-D-glucopyranosyl-(1->6)-beta-D-glucopyranoside (5), dipsacus saponin A (4), and Hederagenin (1) on the classical pathway (CP) of complement system, while the saponins 3-5 did not show the inhibition of hemolysis and rather increase the hemolysis on the alternative pathway (AP).
METHODS AND RESULTS:
However, all of C-3 monodesmosides [prosapogenin CP (2), dipsacus saponin B (6), and dipsacus saponin C (7)] evoked hemolysis directly on the erythrocytes.
Int Immunopharmacol . 2015 Dec;29(2):528-537.
Hederagenin, a major component of Clematis mandshurica Ruprecht root, attenuates inflammatory responses in RAW 264.7 cells and in mice[Pubmed:
26481049]
Abstract
Clematis mandshurica Ruprecht root has been used in Asia as a traditional anti-inflammatory, analgesic, and antitumor agent. Its main active component is Hederagenin, a naturally occurring triterpene, and in this study, we examined the anti-inflammatory effects of Hederagenin in lipopolysaccharide-stimulated RAW 264.7 cells using an enzyme-linked immunosorbent assay, Western blot, and RT-PCR. In addition, its effects on acute inflammation in vivo were observed using a carrageenan-induced mouse hind paw edema assay. Furthermore, the changes on the histopathology and histomorphometry of hind paw skins were examined using carrageenan-treated mice. Treatment with Hederagenin (10, 30 and 100μM) resulted in inhibited levels of protein expression of lipopolysaccharide-stimulated iNOS, COX-2, and NF-κB as well as production of NO, PGE2, TNF-α, IL-1β, and IL-6 induced by lipopolysaccharide. Consistent with these results, Hederagenin also dose-dependently reduced the lipopolysaccharide-induced mRNA levels of iNOS and COX-2, and of the above-mentioned cytokines. Interestingly, results of the carrageenan-induced mouse hind paw edema assay showed an anti-edema effect of Hederagenin. Furthermore, Hederagenin (30mg/kg) inhibited the carrageenan-induced increases in skin thicknesses, infiltrated inflammatory cells, and mast cell degranulation. These results suggest that Hederagenin may possess anti-inflammatory activities.
Keywords: Hederagenin; Histological examination; Inflammatory response; Mouse paw edema; Nuclear factor-kappa B.
Pharm Biol. 2015 Mar;53(3):368-77.
Involvement of norepinephrine and serotonin system in antidepressant-like effects of hederagenin in the rat model of unpredictable chronic mild stress-induced depression.[Pubmed:
25471378]
Previous studies from our laboratory indicated that both acute and subchronic administration of Fructus Akebiae (FAE) [the fruit of Akebiae quinata (Thunb.) Decne, (Lardizabalaceae)] produce antidepressant-like effects in animal depressive behavior tests. FAE contains approximately 70% of Hederagenin (HG) as its main chemical component.
This study compared the antidepressant ability of FAE with that of HG in mice and further investigated the antidepressant-like effects and potential mechanisms of HG in rats subjected to unpredictable chronic mild stress (UCMS).
METHODS AND RESULTS:
Mice received FAE (50 mg/kg) and HG (20 mg/kg) once a day via intragastric administration (i.g.) for 3 weeks. The anxiolytic and antidepressant activities of FAE and HG were compared using elevated plus maze (EPM) and behavioral despair tests including tail suspension test (TST) and forced swimming test (FST), respectively. Antidepressant effects of HG (5 mg/kg) were assessed using the UCMS depressive rat model. Moreover, the levels of monoamine neurotransmitters and relevant gene expression in UCMS rats' hippocampi were determined through high-performance liquid chromatography with electrochemical detection and real-time polymerase chain reaction techniques.
The results of our preliminary screening test suggest that HG at 20 mg/kg, while not FAE at 50 mg/kg, significantly decreased the immobility in both TST and FST compared with the vehicle group when administered chronically; however, there were no significant differences observed between the HG and the FAE group. Chronic administration of HG failed to significantly reverse the altered crossing and rearing behavioral performance, time spent in the open arm and closed entries in the EPM, even if they showed an increased tendency, but HG significantly increased the percent of sucrose preference in the sucrose preference test (SPT) and decreased the immobility time in the FST. HG showed that significant increases of norepinephrine and serotonin levels and exhibited a tendency to increase the expression of 5-hydroxytryptamine (serotonin) 1A receptor mRNA, and to significantly decrease the expression of the mRNA for the serotonin transporter (5-HTT). However, there were no significant differences in the expression of the brain-derived neurotrophic factor.
CONCLUSIONS:
These findings confirm the antidepressant-like effects of HG in a behavioral despair test and UCMS rat model, which may be associated with monoamine neurotransmitters and 5-HTT mRNA expression.
Biochemistry. 2009 Apr 21;48(15):3477-82.
Alpha-hederin, but not hederacoside C and hederagenin from Hedera helix, affects the binding behavior, dynamics, and regulation of beta 2-adrenergic receptors.[Pubmed:
19278262 ]
Hederacoside C, alpha-hederin, and Hederagenin are saponins of dry extracts obtained from the leaves of ivy (Hedera helix L.).
METHODS AND RESULTS:
Internalization of beta(2)-adrenergic receptor-GFP fusion proteins after stimulation with 1 microM terbutaline was inhibited by preincubation of stably transfected HEK293 cells with 1 microM alpha-hederin for 24 h, whereas neither hederacoside C nor Hederagenin (1 microM each) influenced this receptor regulation. After incubation of A549 cells with 5 nM Alexa532-NA, two different diffusion time constants were found for beta(2)AR-Alexa532-NA complexes by fluorescence correlation spectroscopy. Evaluation of the autocorrelation curve revealed diffusion time constants: tau(bound1) = 1.4 +/- 1.1 ms (n = 6) found for receptor-ligand complexes with unrestricted lateral mobility, and tau(bound2) = 34.7 +/- 14.1 ms (n = 6) for receptor-ligand complexes with hindered mobility. The distribution of diffusion time constants was 24.3 +/- 2.5% for tau(bound1) and 8.7 +/- 4.3% for tau(bound2) (n = 6). A549 cells pretreated with 1 microM alpha-hederin for 24 h showed dose-dependent alterations in this distribution with 37.1 +/- 5.5% for tau(bound1) and 4.1 +/- 1.1% for tau(bound2). Simultaneously, the level of Alexa532-NA binding was significantly increased from 33.0 +/- 6.8 to 41.2 +/- 4.6%. In saturation experiments, alpha-hederin did not influence the beta(2)-adrenergic receptor density (B(max)), whereas the K(D) value for Alexa532-NA binding decreased from 36.1 +/- 9.2 to 24.3 +/- 11.1 nM. Pretreatment of HASM cells with alpha-hederin (1 microM, 24 h) revealed an increased intracellular cAMP level of 13.5 +/- 7.0% under stimulating conditions. Remarkably, structure-related saponins like hederacoside C and Hederagenin did not influence either the binding behavior of beta(2)AR or the intracellular cAMP level.
Planta Med. 2000 May;66(4):329-32.
Essential moiety for antimutagenic and cytotoxic activity of hederagenin monodesmosides and bisdesmosides isolated from the stem bark of Kalopanax pictus.[Pubmed:
10865448 ]
METHODS AND RESULTS:
For the elucidation of the antimutagenic and cytotoxic principles from the stem bark of Kalopanax pictus, seven isolated components of this crude drug were tested in the Ames test and the MTT test. Hederagenin and its monodesmosides, kalopanaxsaponin A and I in addition to its bisdesmosides, kalopanaxsaponin B and H, showed potent antimutagenic activities against aflatoxin B1 (AFB1). However, they had no inhibitory effects on mutagenicity induced by the direct mutagen, N-methyl-N'-nitro-N-nitrosoguanidine (MNNG). This suggested that Hederagenin glycosides might effectively prevent the metabolic activation of AFB1 or scavenge the electrophilic intermediate capable of inducing mutation. Hederagenin was found to be an essential moiety for the exhibition of antimutagenicity. Moreover, Hederagenin and its 3-O-glycosides were found to be cytotoxic on various tumor cell lines, P-388, L-1210, U-937, HL-60, SNU-5 and HepG2, while 3,28-di-O-glycosides of Hederagenin were not cytotoxic.
CONCLUSIONS:
Hence, Hederagenin and its 3-O-glycosides could be suitable for cancer treatment chemopreventive drugs.
Langmuir. 2014 Apr 29;30(16):4556-69.
Domain formation and permeabilization induced by the saponin α-hederin and its aglycone hederagenin in a cholesterol-containing bilayer.[Pubmed:
24690040]
Saponins and triterpenic acids have been shown to be able to interact with lipid membranes and domains enriched with cholesterol (rafts). How saponins are able to modulate lipid phase separation in membranes and the role of the sugar chains for this activity is unknown.
METHODS AND RESULTS:
We demonstrate in a binary membrane model composed of DMPC/Chol (3:1 mol/mol) that the saponin α-hederin and its aglycone presenting no sugar chain, the triterpenic acid Hederagenin, are able to induce the formation of lipid domains. We show on multilamellar vesicles (MLV), giant unilamellar vesicles (GUV), and supported planar bilayers (SPB) that the presence of sugar units on the sapogenin accelerates domain formation and increases the proportion of sterols within these domains. The domain shape is also influenced by the presence of sugars because α-hederin and Hederagenin induce the formation of tubular and spherical domains, respectively. These highly curved structures should result from the induction of membrane curvature by both compounds. In addition to the formation of domains, α-hederin and Hederagenin permeabilize GUV. The formation of membrane holes by α-hederin comes along with the accumulation of lipids into nonbilayer structures in SPB. This process might be responsible for the permeabilizing activity of both compounds. In LUV, permeabilization by α-hederin was sterol-dependent.
CONCLUSIONS:
The biological implications of our results and the mechanisms involved are discussed in relation to the activity of saponins and triterpenic acids on membrane rafts, cancer cells, and hemolysis.
Apterin
Catalog No: CFN95005
CAS No: 53947-89-0
Price: $388/10mg
3''-O-Galloylmyricitrin
Catalog No: CFN95057
CAS No: 143202-36-2
Price: $333/5mg
Yuanhuanin
Catalog No: CFN95127
CAS No: 83133-14-6
Price: $318/5mg
3,7,23,24-tetrahydroxycucurbita-5,25-dien-19-al
Catalog No: CFN95168
CAS No: 1446447-97-7
Price: $318/5mg
Polygalin J
Catalog No: CFN95175
CAS No: N/A
Price: $318/5mg
N1,N5,N10-(E)-tri-p-coumaroylspermidine
Catalog No: CFN95256
CAS No: 364368-18-3
Price: $388/10mg
Japondipsaponin E1
Catalog No: CFN95354
CAS No: 175586-66-0
Price: $318/5mg
Foliachinenoside C
Catalog No: CFN95361
CAS No: 1041180-87-3
Price: $368/5mg
Erythro-Guaiacylglycerol-beta-coniferyl aldehyde ether
Catalog No: CFN95416
CAS No: 74474-55-8
Price: $318/5mg
2,11,12-Trihydroxy-7,20-epoxy-8,11,13-abietatriene
Catalog No: CFN95428
CAS No: 1608462-12-9
Price: $318/10mg