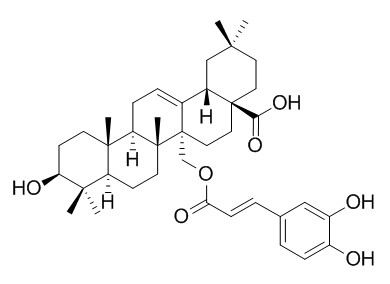
Myriceric acid B
Myriceric acid B is a potent HIV-1 entry inhibitor targeting gp41 and can serve as a lead compound for developing novel anti-HIV-1 drug. Myriceric acid B scavenges DPPH free radicals with IC50 value of 21.8 uM, it inhibits aromatase activity with IC50 value of 6.8 uM, it also exhibits cytotoxic activity towards the MOLT-3 cell line with IC50 values of 3.9 uM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Korean J. Medicinal Crop Sci.2022, 30(2):117-123.
Phytother Res.2022, ptr.7573.
Plants (Basel).2022, 11(16):2126.
Pathol Res Pract.2024, :260:155445.
Molecular & Cellular Toxicology 2024, 00444-8.
Int J Mol Sci.2023, 24(4):3682.
Korean Journal of Pharmacognosy.2020, 51(2):100-106
Biofactors.2018, 44(2):168-179
Oncol Lett.2020, 20(4):122.
J Ethnopharmacol.2020, 249:112381
Related and Featured Products
Eur. J.Org. Chem. 2011, 2011(20-21): 3809-14.
Protoberberine Alkaloids and Cancer Chemopreventive Properties of Compounds from Alangium salviifolium[Reference:
WebLink]
METHODS AND RESULTS:
New protoberberine alkaloids, namely alangiumkaloids A (1) and B (2), 27-O-trans-caffeoylcylicodiscic acid (3), and β-d-glucopyranos-1-yl N-methylpyrrole-2-carboxylate (5) together with Myriceric acid B (4), isoalangiside (6), alangiside (7), 3-O-demethyl-2-O-methylalangiside (8), and demethylalangiside (9) have been isolated from Alangium salviifolium. The cancer chemopreventive properties and cytotoxic activities of the isolated compounds were evaluated.
CONCLUSIONS:
Compounds Myriceric acid B, 4, and 9 scavenged DPPH free radicals with IC50 values of 21.4, 21.8, and 24.0 μm, respectively. Alangisides 7 and 9 inhibited superoxide anion radical formation in the xanthine/xanthine oxidase (XXO) assay with IC50 values of 19.4 and 5.3 μm, respectively. Compounds 6–9 showed excellent antioxidant activity in the oxygen radical absorbance capacity (ORAC) assay with 12.8–24.9 ORAC units. Compounds 3 and Myriceric acid B inhibited aromatase activity with IC50 values of 4.7 and 6.8 μm, respectively. Although the isolated compounds showed only weak cytotoxicity or were inactive, compounds 3 and Myriceric acid B exhibited cytotoxic activity towards the MOLT-3 cell line with IC50 values of 5.6 and 3.9 μm, respectively.
Chinese Pharmacological Bulletin, 2010, 26(4):447-451+452.
The anti-HIV-1 entrance activity and mechanism of action of myriceric acid B from Rhoiptelea chiliantha Diels et Hand-Mazz.[Reference:
WebLink]
To investigate the HIV-1 entry inhibitory activities of Myriceric acid B and C isolated from Rhoiptelea chiliantha Diels et Hand-Mazz and their mechanism of action.
METHODS AND RESULTS:
The plasmids encoding envelope proteins of HIV-1 (pHXB2) and VSV (pVSV-G) were cotransfected 293T cells with pNL4-3. Luc. R-E- to produce HIV-1 Env pseudovirus and VSV-G pseudovirus, respectively, which were used for testing the antiviral activities of these compounds. ELISA and molecular docking were used to study the mechanism of action of the active compounds. Myriceric acid B could significantly inhibit the infection of HIV-1 Env pseudovirus with an IC50 of (8.3 ± 0.2) mg·L-1. The carbonoxyl group at C-28 position and the hydroxyl group at the C-3 position of Myriceric acid B are important for its anti-HIV-1 activity. Like other HIV-1 entry inhibitors targeting gp41 (eg, ADS-J1 and NB-64), Myriceric acid B could also block the gp41 six-helix bundle formation. Molecular docking analysis suggests that Myriceric acid B may bind to the hydrophobic cavity of the gp41 N-trimeric coiled coil.
CONCLUSIONS:
Myriceric acid B is a potent HIV-1 entry inhibitor targeting gp41 and can serve as a lead compound for developing novel anti-HIV-1 drug.