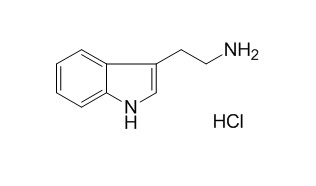
Tryptamine hydrochloride
Tryptamine hydrochloride has antioxidative effects.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Applied Food Research2024, 100662.
Phytochemistry2018, 15:83-92
Biology (Basel).2020, 9(11):363.
Biomol Ther (Seoul).2023, 31(1):40-47.
Spectrochim Acta A2019, 210:372-380
Nutrients.2024, 16(19):3266.
Molecules 2021, 26(4),1092.
J Ethnopharmacol.2024, 318(Pt B):116961.
Univerzita Karlova2022, 228192.
Phytomedicine.2022, 100:154036.
Related and Featured Products
Eiyo to Shokuryo, 1977,30(5): 291-296.
Studies on the Antioxidative Activities of Metabolites of Tryptophan.[Reference:
WebLink]
Antioxidative effects of metabolites of tryptophan (Try) on the autoxidation of oil have been studied little.
METHODS AND RESULTS:
Present paper deals with the antioxidative activities of Try, kynurenine (Kyn), kynurenic acid (Ky. A), xanthorenic acid (Xa. A), anthranilic acid (An. A), 3 or 5-OH-anthranilic acid (3 or 5-OH-An. A), 5-OH-tryptamine (5-OH-Try. Am), Tryptamine hydrochloride (Try. Am-HCl), β-nicotinamide adenine dinucleotide (NAD) and alanine (Ala) using A.O.M., oven test, weighing method test and DPPH method (1, 1-diphenyl-2-picrilhydrozil).
The results obtained were as follows;
1) 3-OH-An. A was the most effective and followed by 5-OH-An. A, Ala, NAD>An. A, Try, Ky. A, Xa. A in decreasing order.
2) 5-OH-An. A showed great antioxidant activity at the concentration of 0.003% by A.O.M. test.
3) Mixture of 3-OH-An. A, 5-OH-An. A, Ala and Ala-NAD was more effective than each of them.
4) The mixture of tocopherol and Try or 5-OH-An. A had a greater antioxidative activity than tocopherol or 5-OH-An. A.
5) When oil was heated for 10hr, antioxidative activity of 5-OH-An. A was decreased, but tocopherol still kept the activity.
The Journal of Chemical Physics, 1983, 78(5):2254-2261.
ENDOR analysis of H-addition radicals and their conversion in irradiated crystals of tryptamine hydrochloride.[Reference:
WebLink]
In a previous study it was shown that the dominant radiation-induced room-temperature radical in single crystals of tryptamine⋅HC1(Tryptamine hydrochloride) is formed by a net H addition to C7 of the indole ring.
METHODS AND RESULTS:
In the present work, it is demonstrated that this species is the precursor for two other indole H-addition radicals. By storage at room temperature, the C7 adduct slowly converts into the C4 adduct. The formation of the C4 adduct could easily be followed by ENDOR. The ENDOR spectra are characterized by hyperfine coupling to six protons bonded to the indole moiety. Upon annealing the crystal above 70 °C, the C7 adduct is more rapidly converted into the C4 adduct, but then the annealing also induces a simultaneous conversion into the C2 adduct. This species also is characterized by six proton hyperfine interactions in the strong-coupling region. The C7 add↠C2 add process could be reversed by exposure to UV light at room temperature, whereas the C7 add↠C4 add process is irreversible upon this procedure. Semiempirical molecular orbital calculations support the interpretations made.
CONCLUSIONS:
The mechanisms of the different processes is discussed in light of the crystal and molecular structure of the compound, and related to similar processes in other compounds, e.g., the purine and pyrimidine bases.