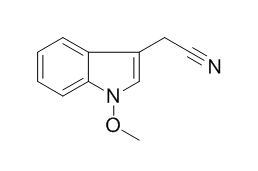
Caulilexin C
Caulilexin C shows inhibitory activity on human Acyl CoA: cholesterol transferase I (hACATI) and on human Acyl CoA: cholesterol transferase 2 (hACAT2) at 100 mug/ml.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Journal of Food Hygiene and Safety2019, 34(5):413-420
VNU Journal of Science2023, 39(2):24-33.
Phytomedicine.2024, 126:155442.
Pharmacogn Mag.2015, 11:S585-91
Journal of Medical Sciences2024, 44(5):p 222-227.
Biol Pharm Bull.2017, 40(6):797-806
Biomol Ther (Seoul).2023, 31(1):40-47.
Cells.2021, 10(11):2919.
Int J Mol Sci.2024, 25(17):9673.
Appl Biochem Biotechnol.2020, 190(2):732-744
Related and Featured Products
Phytochemistry. 2011 Dec;72(18):2308-16.
Interaction of cruciferous phytoanticipins with plant fungal pathogens: indole glucosinolates are not metabolized but the corresponding desulfo-derivatives and nitriles are.[Pubmed:
21920565]
Glucosinolates represent a large group of plant natural products long known for diverse and fascinating physiological functions and activities. Despite the relevance and huge interest on the roles of indole glucosinolates in plant defense, little is known about their direct interaction with microbial plant pathogens.
METHODS AND RESULTS:
Toward this end, the metabolism of indolyl glucosinolates, their corresponding desulfo-derivatives, and derived metabolites, by three fungal species pathogenic on crucifers was investigated. While glucobrassicin, 1-methoxyglucobrassicin, 4-methoxyglucobrassicin were not metabolized by the pathogenic fungi Alternaria brassicicola, Rhizoctonia solani and Sclerotinia sclerotiorum, the corresponding desulfo-derivatives were metabolized to indolyl-3-acetonitrile, Caulilexin C (1-methoxyindolyl-3-acetonitrile) and arvelexin (4-methoxyindolyl-3-acetonitrile) by R. solani and S. sclerotiorum, but not by A. brassicicola. That is, desulfo-glucosinolates were metabolized by two non-host-selective pathogens, but not by a host-selective. Indolyl-3-acetonitrile, Caulilexin C and arvelexin were metabolized to the corresponding indole-3-carboxylic acids. Indolyl-3-acetonitriles displayed higher inhibitory activity than indole desulfo-glucosinolates. Indolyl-3-methanol displayed antifungal activity and was metabolized by A. brassicicola and R. solani to the less antifungal compounds indole-3-carboxaldehyde and indole-3-carboxylic acid.
CONCLUSIONS:
Diindolyl-3-methane was strongly antifungal and stable in fungal cultures, but ascorbigen was not stable in solution and displayed low antifungal activity; neither compound appeared to be metabolized by any of the three fungal species.
The cell-free extracts of mycelia of A. brassicicola displayed low myrosinase activity using glucobrassicin as substrate, but myrosinase activity was not detectable in mycelia of either R. solani or S. sclerotiorum.
Tetrahedron Letters, 2008 , 51 (1) :65-69.
Development of Biologically Active Compounds from Edible Plant Sources XXII. Isolation of Indoles from the Roots of Brassica campestris ssp rapa and their hACAT Inhibitory Activity[Reference:
WebLink]
METHODS AND RESULTS:
The roots of Brassica campestris ssp rapa were extracted with 80% aqueous MeOH, and the concentrated extract was partitioned with EtOAc, n-BuOH and H2O. From the EtOAc fraction, three compounds were isolated through the repeated silica gel and octadecyl silica gel (ODS) column chromatography. From the results of spectroscopic data including NMR and MS, the chemical structures of the compounds were determined as Caulilexin C (1), indoleacetonitrile (2) and arvelexin (3). The arvelexin (3) has been isolated from this plant for the first time.
CONCLUSIONS:
Compounds 1, 2 and 3 showed inhibitory activity on human Acyl CoA: cholesterol transferase I (hACATI) by 54.6 +/- 6.0%, 69.2 +/- 4.7% and 68.6 +/- 3.7%, and on human Acyl CoA: cholesterol transferase 2 (hACAT2) by 4.8 +/- 13.4%, 45.6 +/- 4.8%, and 39.5 +/- 4.3%, respectively, at 100 mu g/ml.