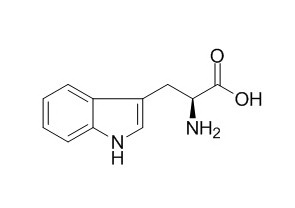
Tryptophan
Tryptophan containing dipeptides are interesting ingredients for functional foods as a natural prevention for hypertension with reduced side effects due to its selective inhibition of the C-domain.Low thalamic Tryptophan uptake appears to be a strong, independent predictor of long survival in patients with previous glioma treatment.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plant Biotechnol (Tokyo).2024, 41(3):267-276.
Front Microbiol.2022, 12:833233.
Cells.2021, 10(11):2919.
J Nat Prod.2021, 84(9):2544-2553.
Trop J Nat Prod Res2023, 7(12):5611-5615.
J.Acta Agriculturae Scandinavica2017, 571-575
J Nat Prod.2022, 85(5):1351-1362.
Natural Product Communications2021, 16(9):1-10.
J Sep Sci.2023, 46(16):e2300160.
J of Ana. Chem.2019, 74(11):1113-1121
Related and Featured Products
Exp Eye Res. 2015 Jan;130:66-72.
Differential tolerance of 'pseudo-pathogenic' tryptophan residues in calcium-binding EGF domains of short fibulin proteins.[Pubmed:
25481286]
An Arg345Trp (R345W) mutation in the last canonical calcium-binding epidermal growth factor (cbEGF) domain of fibulin-3 (F3) causes the rare macular dystrophy, Malattia Leventinese (ML). In cell culture studies, this mutation leads to inefficient F3 secretion and higher intracellular steady state levels, likely due to F3 disulfide bonding and/or protein folding problems. However, how the R345W mutation actually causes ML is still largely unknown.
METHODS AND RESULTS:
Herein we tested whether the introduction of analogous, 'pseudo-pathogenic' Tryptophan mutations immediately after the bn cysteine (bn+1) in other cbEGF domains also caused protein folding/secretion challenges. We found that introduction of Tryptophan mutations into each of the four other F3 canonical cbEGF domains caused a significant reduction in protein secretion ranging from 2.7 to 56% of wild-type (WT) F3 levels. Surprisingly, an R185W mutation in the first canonical cbEGF domain of F3 yielded the highest amount of secretion among the F3 Tryptophan mutants, and its secretion defect could be rescued to near WT levels (95%) after growth temperature reduction. Interestingly, when similarly positioned Tryptophan mutations were introduced into any of the canonical cbEGF domains of the highly homologous protein, fibulin-5 (F5), there was no effect on secretion. In an attempt to make F3 tolerant of Tryptophan residues (like F5), we genetically engineered F3 to have a higher sequence homology with F5 by deleting three insert regions present in F3, but not F5. However, deletion of one or more of these regions did not have a beneficial effect on R345W F3 secretion.
CONCLUSIONS:
Overall, these results demonstrate that the introduction of Tryptophan residues at the bn+1 position does not universally disrupt cbEGF domain folding and secretion, but that their effect is context dependent, and in this case, uniquely disrupt the folding of canonical cbEGF domains of F3, but not F5.
Food Chem. 2015 Jan 1;166:596-602.
Tryptophan-containing dipeptides are C-domain selective inhibitors of angiotensin converting enzyme.[Pubmed:
25053098]
Somatic angiotensin-converting enzyme (ACE) contains two active sites, the C- and N-domain, from which the C-domain is supposed to play a major role in blood pressure regulation and is therefore a promising pharmacological target to reduce blood pressure without side-effects.
METHODS AND RESULTS:
We report for the first time that Tryptophan-containing dipeptides such as Ile-Trp or Val-Trp, which were recently found in food protein hydrolysates, are selective and competitive inhibitors for the C-domain with a selectivity factor of 40 and 70, respectively. Structure-activity studies showed that an N-terminal aliphatic amino acid and a Tryptophan moiety in the P2' position are favourable structures for C-domain inhibition in dipeptides. In contrast, the lactotripeptides Ile-Pro-Pro and Val-Pro-Pro, which were widely used as ingredients for hypotensive food, showed a slight selectivity for the N-domain.
CONCLUSIONS:
Hence, Tryptophan containing dipeptides are interesting ingredients for functional foods as a natural prevention for hypertension with reduced side effects due to its selective inhibition of the C-domain.
J Nucl Med. 2014 Oct;55(10):1605-10.
Clinical significance of tryptophan metabolism in the nontumoral hemisphere in patients with malignant glioma.[Pubmed:
25189339]
METHODS AND RESULTS:
Herein we tested whether the introduction of analogous, 'pseudo-pathogenic' Tryptophan mutations immediately after the bn cysteine (bn+1) in other cbEGF domains also caused protein folding/secretion challenges. We found that introduction of Tryptophan mutations into each of the four other F3 canonical cbEGF domains caused a significant reduction in protein secretion ranging from 2.7 to 56% of wild-type (WT) F3 levels. Surprisingly, an R185W mutation in the first canonical cbEGF domain of F3 yielded the highest amount of secretion among the F3 Tryptophan mutants, and its secretion defect could be rescued to near WT levels (95%) after growth temperature reduction. Interestingly, when similarly positioned Tryptophan mutations were introduced into any of the canonical cbEGF domains of the highly homologous protein, fibulin-5 (F5), there was no effect on secretion. In an attempt to make F3 tolerant of Tryptophan residues (like F5), we genetically engineered F3 to have a higher sequence homology with F5 by deleting three insert regions present in F3, but not F5. However, deletion of one or more of these regions did not have a beneficial effect on R345W F3 secretion.
CONCLUSIONS:
Overall, these results demonstrate that the introduction of Tryptophan residues at the bn+1 position does not universally disrupt cbEGF domain folding and secretion.