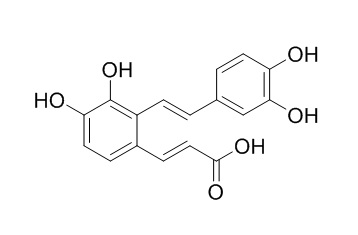
Salvianolic acid F
The role of our hybrid molecules, an analogue of Salvianolic acid F, in compelling the glioma cells towards apoptosis by specifically perturbing the concentration of glutathione along with caspase 6.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2022, 23(13):7115.
Pharmaceuticals (Basel).2022, 15(8):982.
Front Immunol.2017, 8:1542
Appl Microbiol Biotechnol.2018, 102(12):5105-5120
Food Chem Toxicol.2024, 186:114589.
Foods.2023, 12(6):1227.
Int J Mol Sci.2024, 25(23):12733.
Acta Edulis Fungi2020, 27(02):63-76.
Int J Pharm.2022, 618:121636.
ACS Omega.2023, 8(36):32424-32431.
Related and Featured Products
Eur. J. Org. Chem., 2016, 36:5941–9.
Divergent Synthesis of Styryl–Cinnamate Hybrid Analogues Inspired by the Natural Product Salvianolic Acid F as a Premise To Investigate Their Anticancer Activity and Its Metabolomic Profiling[Reference:
WebLink]
The natural product Salvianolic acid F- inspired protecting-group-free synthesis of hydroxylated styryl-cinnamate hybrids (C6-C2-C6-C3 unit) has been achieved by step-economical route via sequential double C-C bond formation in one pot.
METHODS AND RESULTS:
The present method involves multiple reactions (Perkin-condensation/ decarboxylation-Heck cross-coupling reactions) using simple precursors (i.e. hydroxylated benzaldehyde, arylacetic acid and acrylic acid derivatives) in one pot which yields desired unnatural small hybrid molecules (1-12) in varying yields of 35-65%with E-selectivity under microwave irradiation whereas the reported conventional route for synthesis of Salvianolic acid F itself requires six steps with an overall yield of 10.0% besides tedious separation of E/Z isomers that arise from Wittig reaction.Apart from an economical synthesis and products diversity, we herein report the potential of some hybrid molecules (3,8,10 and 11), with the catecholic core, to selectively inhibit glioma cells. The intrinsic mode of action (MOA) for our lead molecule involving caspase 6 and quinonemethide pathway is also reported based on 1HNMR-guided metabolomic profiling.
CONCLUSIONS:
We emphatically demonstrate the role of our hybrid molecules, an analogue of Salvianolic acid F, in compelling the glioma cells towards apoptosis by specifically perturbing the concentration of glutathione along with caspase 6.
(1S,2S)-threo-Honokitriol
Catalog No: CFN95076
CAS No: 1099687-80-5
Price: $413/5mg
3'-Methoxymirificin
Catalog No: CFN95112
CAS No: 1297609-29-0
Price: $318/5mg
Polygalasaponin XLIX
Catalog No: CFN95117
CAS No: 1033593-12-2
Price: $318/10mg
Sibiricose A4
Catalog No: CFN95298
CAS No: 241125-73-5
Price: $318/5mg
Isospinosin
Catalog No: CFN95350
CAS No: 89701-83-7
Price: $318/5mg
8-Hydroxypinoresinol-4'-O-beta-D-glucopyranoside
Catalog No: CFN95356
CAS No: 102582-69-4
Price: $318/5mg
2,11,12-Trihydroxy-7,20-epoxy-8,11,13-abietatriene
Catalog No: CFN95428
CAS No: 1608462-12-9
Price: $318/10mg
12beta-Acetoxy-3beta-hydroxy-7,11,15,23-tetraoxo-lanost-8,20-diene-26-oic acid
Catalog No: CFN95468
CAS No: 1085338-75-5
Price: $318/5mg
Mahuannin J
Catalog No: CFN95533
CAS No: N/A
Price: $318/5mg
3''-O-Caffeoylhesperidin
Catalog No: CFN95564
CAS No: N/A
Price: $318/5mg