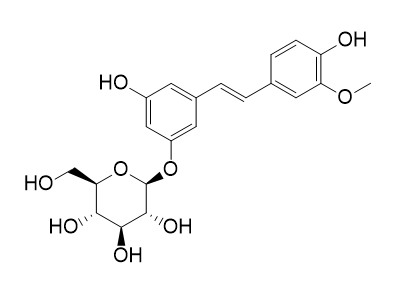
Isorhapontin
Isorhapontin is a stilbenoids compound with antioxidant, antibacterial and antifungal properties.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int. J. Mol. Sci.2022, 23(19), 11900.
J Colloid Interface Sci.2022, 622:298-308.
J Drug Delivery Science and Tech.2022, 67:102957.
Biomedicine & Pharmacotherapy2022, 153:113404.
Phytochem Anal.2016, 27(5):296-303
Int. J. Mol. Sci.2022, 23(8), 4130.
Research Square2021, 10.21203.
Photodermatol Photoimmunol Photomed.2024, 40(1):e12950.
Environ Toxicol.2020, doi: 10.1002
Natural Product Sciences2024, 30(4):254-261.
Related and Featured Products
Int J Food Microbiol . 2013 Jun 3;164(1):99-107.
The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms[Pubmed:
23624538]
The antimicrobial effects of the wood-associated polyphenolic compounds pinosylvin, pinosylvin monomethyl ether, astringin, piceatannol, Isorhapontin, isorhapontigenin, cycloXMe, dHIMP, ArX, and ArXOH were assessed against both Gram-negative (Salmonella) and Gram-positive bacteria (Listeria monocytogenes, Staphylococcus epidermidis, Staphylococcus aureus) and yeasts (Candida tropicalis, Saccharomyces cerevisiae). Particularly the stilbenes pinosylvin, its monomethyl ether and piceatannol demonstrated a clear antimicrobial activity, which in the case of pinosylvin was present also in food matrices like sauerkraut, gravlax and berry jam, but not in milk. The destabilization of the outer membrane of Gram-negative microorganisms, as well as interactions with the cell membrane, as indicated by the NPN uptake and LIVE/DEAD viability staining experiments, can be one of the specific mechanisms behind the antibacterial action. L. monocytogenes was particularly sensitive to pinosylvin, and this effect was also seen in L. monocytogenes internalized in intestinal Caco2 cells at non-cytotoxic pinosylvin concentrations. In general, the antimicrobial effects of pinosylvin were even more prominent than those of a related stilbene, resveratrol, well known for its various bioactivities. According to our results, pinosylvin could have potential as a natural disinfectant or biocide in some targeted applications.
Plant Mol Biol . 2017 Jun;94(3):229-251.
Association genetics of phenolic needle compounds in Norway spruce with variable susceptibility to needle bladder rust[Pubmed:
28190131]
Accumulation of phenolic needle metabolites in Norway spruce is regulated by many genes with small and additive effects and is correlated with the susceptibility against fungal attack. Norway spruce accumulates high foliar concentrations of secondary phenolic metabolites, with important functions for pathogen defence responses. However, the molecular genetic basis underlying the quantitative variation of phenolic compounds and their role in enhanced resistance of spruce to infection by needle bladder rust are unknown. To address these questions, a set of 1035 genome-wide single nucleotide polymorphisms (SNPs) was associated to the quantitative variation of four simple phenylpropanoids, eight stilbenes, nine flavonoids, six related arithmetic parameters and the susceptibility to infection by Chrysomyxa rhododendri in an unstructured natural population of Norway spruce. Thirty-one significant genetic associations for the flavonoids gallocatechin, kaempferol 3-glucoside and quercetin 3-glucoside and the stilbenes resveratrol, piceatannol, astringin and Isorhapontin were discovered, explaining 22-59% of phenotypic variation, and indicating a regulation of phenolic accumulation by many genes with small and additive effects. The phenolics profile differed between trees with high and low susceptibility to the fungus, underlining the importance of phenolic compounds in the defence mechanisms of Norway spruce to C. rhododendri. Results highlight the utility of association studies in non-model tree species and may enable marker-assisted selection of Norway spruce adapted to severe pathogen attack.
Planta Med . 2001 Mar;67(2):158-161.
Inhibition of xanthine and monoamine oxidases by stilbenoids from Veratrum taliense[Pubmed:
11301865]
The bioassay guided refractionation of the methanol extract of roots and rhizomes of Veratrum taliense (Liliaceae) yielded five stilbenoids: veraphenol, resveratrol, piceid, Isorhapontin, and mulberroside E, all inhibiting xanthine oxidase (XO, EC 1.2.3.2.) in vitro in a dose-dependent manner with IC50 values of 11.0, 96.7, 66.1, 70.0, and 78.4 microM, respectively. Veraphenol and mulberroside E were found to be mixed XO inhibitors with the Ki and Ki data of the former being 32.8 and 239.3 microM, and those of latter 32.5 and 13.8 microM, respectively. However, the inhibition on the enzyme by resveratrol, Isorhapontin, and piceid was shown to be competitive with their Ki values of 9.7, 19.1, and 14.3 microM, respectively. Among the five stilbenoids, veraphenol and resveratrol were also revealed to inhibit competitively monoamine oxidase A (MAO, EC 1.4.3.4) with IC50 values at 38.0 and 26.6 microM, and Ki data 36.4 and 47.3 microM, respectively. However, none of the stilbenoids was inhibitory on MAO B in our assay. The structure-activity relationship examination showed that glycosylation of the stilbenoids could reduce the inhibition on XO and diminish the activity against MAO A, indicating that the free phenolic hydroxy group of the compounds was most likely essential for these bioactivities.
Toxicol Appl Pharmacol . 1996 Feb;136(2):381-388.
Wood-derived estrogens: studies in vitro with breast cancer cell lines and in vivo in trout[Pubmed:
8619247]
The wood-derived compound, beta-sitosterol (purity > 90%), was shown to be estrogenic in fish. It induced the expression of the vitellogenin gene in the liver of juvenile and methyltestosterone-treated rainbow trout. Structural similarities to beta-sitosterol notwithstanding, cholesterol, citrostadienol, beta-sitostanol, and 5-androstene-3 beta,17 beta-diol, an estrogenic member of the androstenic steroid group, were inactive. An abietic acid mixture (37% abietic acid, 6% dehydroabietic acid, and a remainder of unknown compounds) showed slight hormonal activity in feed, but it was completely inactive when given intraperitoneally in implants. The estrogenic component of the abietic acid preparation was not identified. In addition, to beta-sitosterol and abietic acid, several other wood-derived compounds including betulin, isorhapontigenin, Isorhapontin, and pinosylvin were estrogenic in breast cancer cells (MCF-7 or T-47D). However, betulin and pinosylvin, available in sufficient amounts for in vivo testing, did not induce the expression of the vitellogenin gene. Differences in the primary sequences of human and fish estrogen receptors (hormone as well as DNA-binding regions) or uptake and metabolism of the compounds may explain the discrepancy between the two estrogen bioassays. Wood-derived compounds such as beta-sitosterol, present in pulp and paper mill effluents, may account for the weak estrogenicity of debarking effluent seen at the vitellogenin expression bioassay.
Phytochem Anal . Nov-Dec 2014;25(6):529-536.
Rapid chemical characterisation of stilbenes in the root bark of Norway spruce by off-line HPLC/DAD-NMR[Pubmed:
24777944]
Introduction: Stilbenes are plant secondary metabolites that have shown promising and varied biological activities. Stilbenes are presently actively studied for the exploitation of this primary raw material resource, involving the concept of biorefining. Methods for the rapid discovery of new and known stilbene structures from various plant sources are thus keenly sought.
Objective: To establish a simple and rapid technique of off-line HPLC with a diode-array detector (DAD) and NMR for the unambiguous structural elucidation of stilbene structures in the root bark of Norway spruce [Picea abies (L.) Karst.].
Material and methods: The stilbene containing fraction was extracted from the plant bark with an ethanol:water mixture (95:5, v/v) preceded by defatting of hydrophobic compounds with n-hexane using the accelerated solvent extraction technique. A portion of the ethanol-water soluble extract was hydrolysed with β-glucosidase to prepare stilbene aglycones. The extracts were further purified and enriched using a polymeric adsorbent. Stilbene-enriched extracts were directly characterised by off-line HPLC/DAD-NMR in conjunction with HPLC/DAD and HPLC/DAD with electrospray ionisation MS(n).
Results: Trans-Isorhapontin and trans-astringin were identified as the major, and trans-piceid as a minor, stilbene glucosides of the bark of roots of Picea abies. Not only stilbene glucosides but also the corresponding stilbene aglycones, such as trans-resveratrol, trans-piceatannol and trans-isorhapontigenin, were rapidly identified from the hydrolysed extract. The acquired heteronuclear single-quantum coherence and heteronuclear multiple bond correlation spectra were used to assign the complete carbon NMR chemical shifts of trans-Isorhapontin and trans-astringin without the need of acquiring a (13)C-NMR spectrum.
Conclusion: The off-line HPLC/DAD-NMR method is expedient for the unambiguous identication of structurally similar stilbenes in plant extracts.