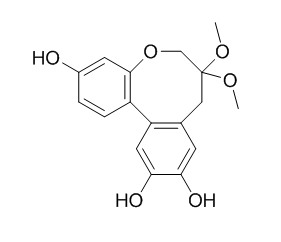
Protosappanin A dimethyl acetal
Protosappanin A dimethyl acetal shows significant xanthine oxidase inhibitory activity in a concentration-dependent manner.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2019, 24(24),4583
Planta Med.2022, a-1876-3009.
Molecules.2019, 24(16):E3003
Life Sci.2018, 209:498-506
Food Hydrocolloids2024, 156:110345
J Biochem.2024, 175(3):253-263.
Front Chem.2023, 11:1245071.
ACS Nano.2023, 17(11):9972-9986.
J of Pharmaceutical Analysis2020, doi: 10.1016
Eur J Pharmacol.2023, 950:175772.
Related and Featured Products
Chem Pharm Bull (Tokyo). 2005 Aug;53(8):984-8.
Xanthine oxidase inhibitors from the heartwood of Vietnamese Caesalpinia sappan.[Pubmed:
16079532]
METHODS AND RESULTS:
From the MeOH extract of Vietnamese Caesalpinia sappan, a novel biogenetically exclusive benzindenopyran, with a new carbon framework, neoprotosappanin (1), and a new compound, Protosappanin A dimethyl acetal (3), were isolated together with protosappanin E-2 (2), neosappanone A (4), and 13 previously reported phenolic compounds (5-17). Their structures were elucidated on the basis of spectroscopic data. Compounds 1-4, 7, 13, and 15-17 showed significant xanthine oxidase inhibitory activity in a concentration-dependent manner, and sappanchalcone (17) showed the most potent activity with an IC50 value of 3.9 microM, comparable to that of positive control allopurinol (IC50, 2.5 microM).
CONCLUSIONS:
The kinetic study of these inhibitors indicated that they are competitive inhibitors, the same as allopurinol, except for 1 and 16 which are noncompetitive inhibitors.