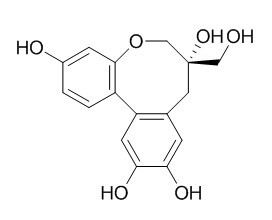
Protosappanin B
Protosappanin B possesses antitumor, anti-inflammation and anti-oxidation properties, it protects PC12 cells against oxygen–glucose deprivation-induced neuronal death by maintaining mitochondrial homeostasis via induction of ubiquitin-dependent p53 protein degradation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2019, 10:1226
Plants (Basel).2021, 10(11):2317.
Evid Based Complement Alternat Med.2021, 2021:5023536.
Plants2022, 11(3),294.
Molecules.2021, 26(9):2791.
Evid Based Complement Alternat Med.2017, 2017:1583185
Chem Pharm Bull (Tokyo).2019, 67(11):1242-1247
Allergol Immunopathol (Madr).2022, 1;50(4):23-30.
Evid Based Complement Alternat Med.2021, 2021:6687513.
Fitoterapia.2024, 175:105955.
Related and Featured Products
Integr Cancer Ther. 2016 Mar;15(1):87-95.
Antitumor Effects of Purified Protosappanin B Extracted From Lignum Sappan.[Pubmed:
26036624 ]
To assess the antitumor effects of Protosappanin B extracted from Lignum Sappan.
METHODS AND RESULTS:
Lignum Sappan was sequentially extracted by boiling water and ethyl acetate. The resulting extract was separated by column chromatography, to yield Protosappanin B. The compound was then identified by thin-layer chromatography, high-performance liquid chromatography, elemental analysis, and spectrometry (infrared and ultraviolet). The effects on tumor cell viability and growth of purified Protosappanin B were evaluated in vitro by trypan blue exclusion and MTT assays, respectively. And the effects of Protosappanin B were assessed in vivo, on H22 mouse liver cancer cell invasion and the survival of tumor-bearing mice.
Protosappanin B (2 mg/mL) reduced the viability of human bladder cancer T24 cells and mouse bladder cancer BTT cells in a time-dependent manner (P < .05) and significantly inhibited the growth of the human colon cancer cell lines HCT-116 and SW-480. IC50 values of 21.32, 26.73, and 76.53 µg/mL were obtained for SW-480, HCT-116, and BTT cells, respectively, after 48 hours of treatment with Protosappanin B. In addition, pretreatment of H22 cells with Protosappanin B (final concentration = 6.25 mg/mL) resulted in complete inhibition of tumor formation in KM mice. Furthermore, Protosappanin B (200 and 300 mg/kg) significantly increased the survival of BTT tumor-bearing T739 mice, at a rate comparable to that of 1 mg/kg mitomycin.
CONCLUSIONS:
Protosappanin B extracted from Lignum Sappan exerts marked antitumor effects both in vitro and in vivo.
J Ethnopharmacol. 2013 Jul 9;148(2):682-90.
An LC/MS/MS method for simultaneous quantitation of two homoisoflavones: protosappanin B and brazilin with hypoglycemic activity in rat plasma and its application to a comparative pharmacokinetic study in normal and streptozotocin-treated rats.[Pubmed:
23707335]
The heartwood of Caesalpinia sappan L. (Leguminosae), a widely used Chinese medicine in folk, has been used for the treatment of traumatic injury, stasis pain, amenorrhea, dysmenorrheal, as well as stabbing pain in the chest, abdomen and so on. Protosappanin B and brazilin, as the major bioactive homoisoflavones of Sappan Lignum, are used as the marker components for the quality control of the herb in China Pharmacopoeia. To establish a sensitive LC/MS/MS method for investigating the pharmacokinetic properties of Protosappanin B and brazilin in rats after oral administration of Sappan Lignum extract, and compare their pharmacokinetics difference between normal and streptozotocin-treated rats.
METHODS AND RESULTS:
A rapid, selective and sensitive LC/MS/MS method was developed and validated for the simultaneous quantification of Protosappanin B and brazilin in rat plasma. Normal and streptozotocin-treated rats were orally administered with the Sappan Lignum extract at the same dose of 2.83 g extract/kg body weight (equivalent to 35.56 mg/kg of Protosappanin B and 52.25 mg/kg of brazilin), respectively. After oral administration of Sappan Lignum extract, a remarkable increase (p<0.05) in the value of AUC0-24h, AUC0-∞, Cmax and T1/2 associated with Protosappanin B and brazilin was observed in the streptozotocin-treated group. Compared with the normal rats, elimination of both compounds in the streptozotocin-treated rats was slower.
CONCLUSIONS:
The established method was successfully applied to compare the pharmacokinetic behaviors of Protosappanin B and brazilin in rat plasma after oral administration of Sappan Lignum extract between normal and streptozotocin-treated groups; the results might suggest the accumulation of both compounds in diabetic pathologic states and the adverse reaction should be considered when it was used.
Eur J Pharmacol. 2015 Mar 15;751:13-23.
Protosappanin B protects PC12 cells against oxygen-glucose deprivation-induced neuronal death by maintaining mitochondrial homeostasis via induction of ubiquitin-dependent p53 protein degradation.[Pubmed:
25657114 ]
Protosappanin B (PTB) is a bioactive dibenzoxocin derivative isolated from Caesalpinia sappan L.
METHODS AND RESULTS:
Here, we investigated the neuroprotective effects and the potential mechanisms of Protosappanin B on oxygen-glucose deprivation (OGD)-injured PC12 cells. Results showed that Protosappanin B significantly increased cell viability, inhibited cell apoptosis and up-regulated the expression of growth-associated protein 43 (a marker of neural outgrowth). Moreover, our study revealed that Protosappanin B effectively maintained mitochondrial homeostasis by up-regulation of mitochondrial membrane potential (MMP), inhibition of cytochrome c release from mitochondria and inactivation of mitochondrial caspase-9/3 apoptosis pathway. Further study showed that Protosappanin B significantly promoted cytoplasmic component degradation of p53 protein, a key negative regulator for mitochondrial function, resulting in a release of Bcl-2 from p53-Bcl-2 complex and an enhancing translocation of Bcl-2 to mitochondrial outer membrane. Finally, we found the degradation of p53 protein was induced by Protosappanin B via activation of a MDM2-dependent ubiquitination process.
CONCLUSIONS:
Taken together, our findings provided a new viewpoint of neuronal protection strategy for anoxia and ischemic injury with natural small molecular dibenzoxocin derivative by activating ubiquitin-dependent p53 protein degradation as well as increasing mitochondrial function.
Biol Pharm Bull. 2007 Jan;30(1):193-6.
In vitro study for inhibition of NO production about constituents of Sappan Lignum.[Pubmed:
17202686]
METHODS AND RESULTS:
As it was reported that Brazilin inhibited inducible NO gene, we conducted to similar tests for six known compounds isolated from Sappan Lignum, namely, brazilein, sappanchalcone, protosappanin A, Protosappanin B, protosappanin C besides brazilin. And six compounds were also subjected to six tests to speculate their properties: (1) inhibition of NO production by cultured J774.1 (macrophage-like) cell line, (2) suppression of inducible NO synthase (iNOS) gene expression, (3) inhibition of NO production by murine peritoneal macrophages, (4) DPPH radical scavenging activity, (5) reduction of ferric ion and (6) antioxidant activity. Brazilein and sappanchalcone showed significant inhibition of lipopolysaccharide (LPS)-induced NO production by J774.1 cell line like Brazilin; 100% inhibition at 30 microM in test (1) and at 10 microM in test (3). The mechanisms underlying the inhibition of NO production by the compounds were investigated in test (2). As a result, brazilin was found to almost completely suppress iNOS gene expression at 100 microM as reported, and brazilein and sappanchalcone also suppressed iNOS gene expression. But strong activities were not observed for protosappanins A, B and C. So, we conducted tests (4), (5) and (6) to investigate other properties about six compounds. Protosappanin A and Brazilin demonstrated high antioxidant activity compared with Vitamin E in tests (4) and (5). Protosappanin A and B inhibited the oxidation of linoleic acid in test (6). Among the dibenzoxocin derivatives, only protosappanin C did not show significant activity in all the tests.
CONCLUSIONS:
We found that sappanchalcone showed same activity as brazilin, and six compounds isolated from Sappan Lignum showed various properties.