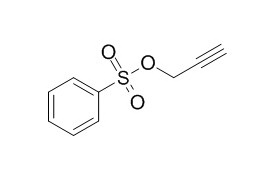
Propargyl benzenesulfonate
Propargyl benzenesulfonate improves the SEI formation process.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Br J Pharmacol.2016, 173(2):396-410
Food Chem.2018, 262:78-85
VNU J of Science: Med.&Pharm. Sci.2023, 39(1):20-29.
Fitoterapia.2018, 124:92-102
Drug Dev Res.2020, doi: 10.1002
J Food Sci Technol.2019, 56(5):2712-2720
Foods.2023, 12(2):318.
J Appl Biol Chem2021, 64(3):245-251.
Environ Toxicol.2019, 34(12):1354-1362
Plants (Basel).2020, 9(11):1422.
Related and Featured Products
214th ECS Meeting, Abstract #1204, © The Electrochemical Society
Propargyl Benzenesulfonate – A Triple-Bonded Electrolyte Additive for Electrolytes in Lithium Ion Batteries.[Reference:
WebLink]
METHODS AND RESULTS:
We focused on a triple-bonded compound, Propargyl benzenesulfonate (S1), which influences the SEI formation process in ethylene carbonate, diethylene carbonate (EC/DEC)- and propylene carbonate (PC)- based electrolytes. In contrast to many other additives Propargyl benzenesulfonate decomposes in a potential range of 1200 mV to 1400 mV against Li/Li+. This fact is very meaningful because S1 provides SEI formation before we reach the edge of the potential window of carbonate based electrolytes. K. Abe et al. already investigated a compound called propargyl methanesulfonate. By the substitution of the methyl-group for a phenyl-group we found a compound which is commercially available and in the comparison cheaper. Propargyl benzenesulfonate was surveyed by cyclovoltammetry, constant current cycling and in situ Fourier transform infrared (FTIR) spectroscopy in a specially developed IR cell.
CONCLUSIONS:
It was concluded that Propargyl benzenesulfonate improves the SEI formation process. By the obtained IR data a possible reaction mechanism of Propargyl benzenesulfonate in the electrolyte was investigated.
3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No: CFN95021
CAS No: 1178-24-1
Price: $268/10mg
Momordicine V
Catalog No: CFN95164
CAS No: 1012315-36-4
Price: $463/5mg
Corymboside
Catalog No: CFN95292
CAS No: 73543-87-0
Price: $318/10mg
8'-O-(3-hydroxy-3-methylglutaryl)-8'-hydroxyabscisic acid
Catalog No: CFN95308
CAS No: 69790-31-4
Price: $318/5mg
New compound 7
Catalog No: CFN95330
CAS No: N/A
Price: $368/5mg
Melitidin
Catalog No: CFN95419
CAS No: 1162664-58-5
Price: $318/10mg
Pinocembrin 7-O-(4'',6''-hexahydroxydiphenoyl)-beta-D-glucose
Catalog No: CFN95462
CAS No: 1825287-22-6
Price: $318/10mg
Quercetin 3,5-O-diglucoside
Catalog No: CFN95486
CAS No: 206257-35-4
Price: $368/10mg
12beta-Acetoxy-7beta-hydroxy-3,11,15,23-tetraoxo-5alpha-lanosta-8,20-dien-26-oic acid
Catalog No: CFN95515
CAS No: 1245946-62-6
Price: $318/5mg
Octyl 3-(4-hydroxyphenyl)prop-2-enoate
Catalog No: CFN95563
CAS No: N/A
Price: $318/5mg