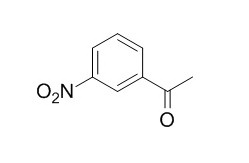
3'-Nitroacetophenone
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2019, 24(21):E3834
Food Science and Preservation2024, 31(3):486-498.
Bull.Natl.Mus.Nat.Sci.,Ser.B.2024, 50(2):79¨C86
Pharmacogn J.2022, 14(2):350-357
Horticulture, Environment, and Biotechnology2025, 66:729-739.
J-STAGE2015, 249-255
Methods Protoc.2024, 7(6):95.
Antioxidants2022, 11(2),234.
Int J Mol Sci.2021, 22(21):11447.
Korean J. of Food Sci. and Tech2016, 172-177
Related and Featured Products
Applied Catalysis A: General, 2013, 462-463:121-128.
Hydrogenation of 3-nitroacetophenone over rhodium/silica catalysts: Effect of metal dispersion and catalyst support[Reference:
WebLink]
METHODS AND RESULTS:
The effect of physical properties of rhodium/silica catalysts on the hydrogenation of 3-nitroacetophenone was investigated using a series of catalysts with different particle sizes, pore sizes and dispersions. The hydrogenation reaction was carried out in the liquid phase at different temperatures (313–343 K) and hydrogen pressures (2–5 barg). The hydrogenation of 3-nitroacetophenone was found to be a consecutive reaction with 3-aminoacetophenone, 1-(3-aminophenyl) ethanol, and 1-(3-aminocyclohexyl) ethanol the main products; traces of 3-aminoaniline were also detected. The reaction was zero order with respect to the concentration of 3-nitroacetophenone and half order with respect to hydrogen. The rate of formation and disappearance of intermediates increased with increasing temperature and hydrogen pressure. The rate of ring hydrogenation to form 1-(3-aminocyclohexyl) ethanol shows a marked increase at high temperatures. The catalytic activity varies with both the pore size of silica support and the rhodium dispersion with no significant influence of catalyst particle size. The turnover frequency increases with decreasing metal dispersion (or with increasing average metal crystallite size) revealing an antipathetic particle size effect.
CONCLUSIONS:
This behaviour can be related to changes in the electronic and structural make up of small metal crystallites but suggests that the reaction takes place on the plane face surface of the FCC close-pack structure of rhodium in which the number of face surface atoms increases at the expense of the edge and corner sites as the metal crystallite size increases.