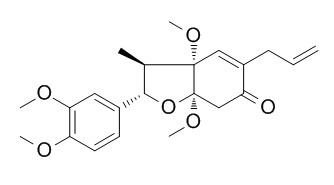
Piperenone
Piperenone is an insect antifeeding substance. It has anti-platelet-activating factor(PAF) activities, is a PAF-acether antagonist.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Microbiol.2022, 13:835463.
Prev Nutr Food Sci.2024, 29(4):504-511.
J Pharm Anal.2016, 6(6):363-373
J Korean Med Ophthalmol Otolaryngol Dermatol2023, 36(1):21-39.
Immunopharmacol Immunotoxicol.2024, 46(4):496-508.
Mol Neurobiol.2021, 58(8):3665-3676.
J Ethnopharmacol.2023, 321:117501.
Green Chemistry2021, ISSUE 2.
Food Science and Human Wellness2022, 11(4):965-974
Int J Mol Sci.2024, 25(16):8846.
Related and Featured Products
Pharmacol Res Commun. 1986 Aug;18 Suppl:25-32.
Crystal and molecular structure of BN 52021, a PAF-acether antagonist. Comparison with the conformation of Kadsurenone and related compounds.[Pubmed:
3774849]
METHODS AND RESULTS:
BN52021 (Ginkgolide B) monohydrate C20H24O10 X H2O crystallizes in the triclinic space group P1 with two independant molecules in the unit cell of which parameters are a = 7.627(2), b = 11.514(3), c = 12.941(3)A, alpha = 97.05(2), beta = 90.27(2) and gamma = 108.71(2)o, V = 1067.06A3. Calculated density Dx = 1.320g X cm-3. The crystal structure was solved by Patterson methods starting from dreiding model data. The final reliability index R = 0.099 for 3848 observed reflections collected with a four-circle diffractometer.
CONCLUSIONS:
Anti-PAF activities of Kadsurenone, Kadsurin-A, Kadsurin-B and Piperenone, molecular structure of MIRANDIN-A, related compounds and BN52021 suggest that a distance 0-0 of about 6.6A observed in Kadsurenone and in BN52021 could be favourable to anti-PAF activity.
Agricultural & Biological Chemistry,1976, 40(6):1113-8.
The structure of piperenone[Reference:
WebLink]
METHODS AND RESULTS:
The structure of a new neolignan, Piperenone, isolated as an insect antifeeding substance from Piper futokadzura Sieb. et Zucc. has been determined on the basis of chemical and spectral evidence. It is shown to be 5-allyl-3a, 7a-dimethoxy-2-(3, 4-dimethoxyphenyl)-3-methyl-2, 3, 3a, 6, 7, 7a-hexahydro-6-oxobenzofuran (I). Both the absolute configurations at C-2 and C-3, two of four asymmetric carbons in I are deduced to be S-arrangements.
Acta Chemica Scandinavica,1995,49 (2):142-8.
Neolignans from Piper schmidtii and reassignment of the structure of schmiditin.[Reference:
WebLink]
METHODS AND RESULTS:
A new neolignan, (7R,8S,1'S)-A8'-1',4'-dihydro-3,4,5'-trimethoxy-4'-oxo-8.1',7.0.2' -lignan [(2R,3S,3aS)-2-(3,4-dimethoxyphenyl)-3,3a-dihydro-5-methoxy-3-methyl-3a-(2-propenyl)-2-benzofuran-6(2H)-one] (1), together with five known neolignans and a known alkaloid were isolated from the stems of Piper schmidtii Hook f. The known compounds were identified as kadsurin B (3), Piperenone (4), (7R,8S, 1'S)-A 8' -1',4'-dihydro-5' -methoxy- 3,4-methylenedioxy-4'oxo-8.1',7.0.2'-lignan (5), lancifolin C (6), lancifolin D (7) and an alkaloid, 1-cinnamoylpyrrolidine (2). The earlier proposed structure of schmiditin (12) was revised to (7S,8R,3'S,4'R,6'S)-88'-3',4',5',6'-tetrahydro-6' -hydroxy-3',4'dimethoxy-3,4- methylenedioxy-8.3',7.0.4' -lignan [(2S,3R,3aS,6S,7aR)-2-(l,3-benzodioxol-5-yl)- 2,3,3a,6,7,7a-hexahydro-3a,7a-dimethoxy-3-methyl-5-(2-propenyl)-2-benzofuran-6-ol= kadsurin B] (3) on the basis of spectral data and chemical transformations.
CONCLUSIONS:
Reassignment of the 1H NMR data of lancifolin C (6), the absolute stereochemistry of kadsurin A (10) and 13C NMR data of Piperenone (4) and lancifolin C (6) are also reported. In addition, methyl piperate (8) was isolated from the fruits.