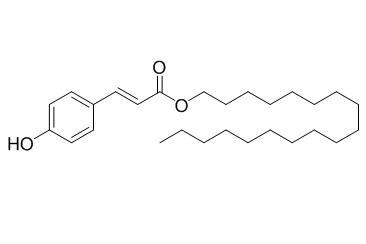
Octadecyl p-coumarate
A mixture of (E)-octadecyl p-coumarate and (Z)-octadecyl p-coumarate has antifungal activity , it shows the dose dependent inhibition of the spore germination of Alternaria alternata and A. porri.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Onco Targets Ther.2017, 10:3467-3474
Int J Cosmet Sci.2019, 41(1):12-20
Food Chem.2021, 337:128023.
J Agric Food Chem.2022, 70(51):16176-16187.
RSC Advances2017, 86
Front Chem.2024, 12:1385844.
J Drug Delivery Science and Tech.2022, 67:102957.
Fitoterapia.2015, 100:179-86
Antioxidants (Basel).2020, 9(2): E119
Molecules.2023, 28(13):4971.
Related and Featured Products
Nat Prod Commun. 2011 Dec;6(12):1889-92.
Antifungal activity and isomerization of octadecyl p-coumarates from Ipomoea carnea subsp. fistulosa.[Pubmed:
22312731]
METHODS AND RESULTS:
Bioassay monitored HPLC assisted isolation and purification of the chief antifungal fraction of the leaves of Ipomoea carnea subsp. fistulosa (Convulvulaceae) were achieved using Colletotrichum gloeosporioides and Cladosporium cucumerinum as test organisms. The activity of the purified fraction was further confirmed by the dose dependent inhibition of the spore germination of Alternaria alternata and A. porri. The active fraction was identified as a mixture of (E)-Octadecyl p-coumarate and (Z)-Octadecyl p-coumarate. The two isomers were detected on an HPLC column with substantially different retention times, but once eluted from the column, one form was partly converted to the other in daylight.
CONCLUSIONS:
Conclusive evidence for the structures and their isomerization were obtained from the HPLC behavior, IR, UV, HRESIMS, CIMS and and NMR spectral data. Important 1H NMR and 13C NMR signals could be separately assigned for the isomers using 2D NMR techniques.
Zhongguo Zhong Yao Za Zhi. 2008 Mar;33(5):524-6.
Chemical constituents from Hedyotis diffusa.[Pubmed:
18536374]
To investigate the chemical constituents from Hedyotis diffusa.
METHODS AND RESULTS:
The compounds were isolated and purified by various chromatographic techniques and identified by their physicochemical properties and spectral data. Eight compounds were isolated and identified as octadecyl (E)-p-coumarate ((E)-Octadecyl p-coumarate,1), p-E-methoxy-cinnamic acid (2), ferulic acid (3), scopoletin (4), succinic acid (5), aurantiamide acetate (6), rubiadin (7), robustaquinone D (8).
CONCLUSIONS:
Compounds 1-8 were obtained from genus Hedyotis for the first time.