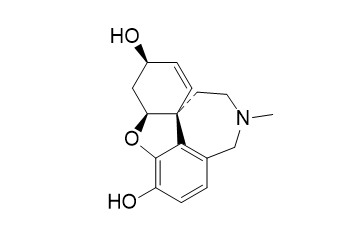
O-Desmethyl galanthamine
O-Desmethyl Galanthamine (Sanguinine) is galanthamine-type alkaloid. O-Desmethyl Galanthamine is an acetylcholinesterase (AChE) inhibitor, with an IC50 1.83 μM. Sanguinine only shows activity against the fibroblast lineage.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Semyung University2017, 149407
Cell Rep.2020, 32(11):108158.
SCOPUS.2020, 836-847.
J Ethnopharmacol.2020, 269:113752.
Eur J Pharmacol.2023, 960:176121.
Pharmaceuticals (Basel).2024, 18(1):19.
Nutr Metab (Lond).2019, 16:31
J Med Food.2021, 24(3):209-217.
Tropical Journal of Pharmaceutical Research 2021, 20(6):1165-1170.
Arch Biochem Biophys.2018, 644:93-99
Related and Featured Products
Phytochemistry . 2020 Jul;175:112390.
Gigantelline, gigantellinine and gigancrinine, cherylline- and crinine-type alkaloids isolated from Crinum jagus with anti-acetylcholinesterase activity[Pubmed:
32335411]
Three undescribed Amarylidaceae alkaloids, named gigantelline, gigantellinine and gigancrinine, were isolated from Crinum jagus (syn. = Crinum giganteum) collected in Senegal, together with the already known sanguinine, cherylline, lycorine, crinine, flexinine and the isoquinolinone derivative hippadine. Gigantelline, gigantellinine and gigancrinine were characterized as 4-(6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydro-isoquinolin-4-yl)-phenol, its 7-O-demethyl-5ꞌ-hydroxy-4ꞌ-methoxy derivative and 5,6a,7,7a,8a,9-hexahydro-6,9a-ethano[1,3]dioxolo[4,5-j]oxireno[2,3-b]phenanthridin-9-ol, respectively, by using spectroscopic (1D and 2D 1H and 13C NMR and HRESIMS) and chemical methods. Their relative configuration was assigned by NOESY NMR spectra and NMR calculations, while the absolute configuration was assigned using electronic circular dichroism (ECD) experiments and calculations. Sanguinine, cherylline, crinine, flexinine, and the isoquinolinone hippadine, were isolated for the first time from C. jagus. Cherylline, gigantellinine, crinine, flexinine and sanguinine inhibited the activity of AChE in a dose-dependent manner, and inhibition by sanguinine was remarkably effective (IC50 = 1.83 ± 0.01 μM). Cherylline and hippadine showed weak cytotoxicity at 100 μM.
Pharmacogenetics . 1999 Dec;9(6):661-668.
The O-demethylation of the antidementia drug galanthamine is catalysed by cytochrome P450 2D6[Pubmed:
10634129]
Galanthamine proved effective in symptomatic treatment of senile dementia of Alzheimer's type. The aim of this study was to elucidate the metabolism of galanthamine. Two novel metabolites of galanthamine have been isolated from the urine of eight young men after single doses of 10-15 mg. Some 19.8% of the doses were excreted as O-demethylgalanthamine glucuronide, 5% as N-demethylgalanthamine, 25.1% as galanthamine, and 0.8% as epigalanthamine. After coadministration of quinidine hydrogen sulfate, which inhibits cytochrome P450 2D6 (CYP2D6) selectively, O-demethylgalanthamine glucuronide was highly diminished in urine. In vitro, human liver microsomes metabolized galanthamine to O-demethylgalanthamine with Vmax 5.2 nmol/mg protein/h and Km 187 microM. Ki of quinidine to inhibit O-demethylation was 28 nM. To inhibit cholinesterases, O-demethylgalanthamine was 10-fold more selective for acetylcholinesterase (AChE) versus butyrylcholinesterase (BuChE) than galanthamine. After glucuronidation, O-demethylgalanthamine failed to inhibit AChE and BuChE. N-Demethylgalanthamine inhibited cholinesterases less potently than galanthamine.
Anticancer Agents Med Chem . 2019;19(5):707-717.
Cytotoxic and Genotoxic Activities of Alkaloids from the Bulbs of Griffinia gardneriana and Habranthus itaobinus (Amaryllidaceae)[Pubmed:
30657047]
Background: Amaryllidaceae plants are known to be a great source of alkaloids, which are considered an extensive group of compounds encompassing a wide range of biological activities. The remarkable cytotoxic activities observed in most of the Amaryllidaceae alkaloids derivatives have prompt the chemical and biological investigations in unexplored species from Brazil.
Objective: To evaluate the cytotoxic and genotoxic properties of alkaloids of Griffinia gardneriana and Habranthus itaobinus bulbs and study the role of caspase-3 as a molecular apoptosis mediator.
Methods: Methanolic crude extracts of Griffinia gardneriana and Habranthus itaobinus bulbs were submitted to acid-base extraction to obtain alkaloid-enriched fractions. The obtained fractions were fractionated using chromatographic techniques leading to isolation and identification of some alkaloids accomplished via HPLC and 1H-NMR, respectively. Molecular docking studies assessed the amount of free binding energy between the isolated alkaloids with the caspase-3 protein and also calculated the theoretical value of Ki. Studies have also been developed to evaluate in vitro cytotoxicity and genotoxicity in such alkaloids and apoptosis activation via the caspase pathway using both tumor and normal cell lines.
Results: Seven alkaloids were isolated and identified. Among these, 11-hydroxyvittatine and 2-α-7- dimethoxyhomolycorine were not cytotoxic, whereas tazettine, trisphaeridine, and sanguinine only showed activity against the fibroblast lineage. Lycorine and pretazettine were 10 to 30 folds more cytotoxic than the other alkaloids, including cancerous lines, and were genotoxic and capable of promoting apoptosis via the caspase-3 pathway. This result supports data obtained in docking studies wherein these two compounds exhibited the highest free energy values.
Conclusion: The cytotoxicity assay revealed that, among the seven alkaloids isolated, only lycorine and pretazettine were active against different cell lines, exhibiting concentration- and time-dependent cytotoxic actions alongside genotoxic action and the ability to induce apoptosis by caspase-3, a result consistent with those obtained in docking studies.
Life Sci . 2002 Oct 11;71(21):2521-2529.
Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts[Pubmed:
12270757]
Amaryllidaceous plants produce pharmacologically active alkaloids, galanthamine being the most interesting for its use in the treatment of Alzheimer's disease as a cholinesterase inhibitor. The aim of this work was to test 23 pure Amaryllidaceae alkaloids and 26 extracts from different species of the genus Narcissus for their acetylcholinesterase inhibitory activity using galanthamine as a reference. Only seven alkaloids, belonging to the galanthamine and lycorine skeleton types, exhibited such an effect, sanguinine being the most active, even more than galanthamine. All the extracts with the highest acetylcholinesterase inhibitory activity contained galanthamine except that of N. assoanus, a lycorine type alkaloid-bearing species.
Nat Prod Commun . 2014 Feb;9(2):159-162.
Alkaloids from Habranthus tubispathus and H. jamesonii, two amaryllidaceae with acetyl- and butyrylcholinesterase inhibition activity[Pubmed:
24689279]
Alzheimer's disease (AD) is a neurodegenerative disorder associated with memory impairment and cognitive deficit. Most of the drugs currently available for the treatment of AD are acetylcholinesterase (AChE) inhibitors. Plants of the Amaryllidaceae family are known to synthesize alkaloids, which have shown AChE inhibitory activity. Habranthus tubispathus and H. jamesonii are two Amaryllidaceae that can be found growing wild to the southwest of Buenos Aires in Argentina. Acetyl- and butyrylcholinesterase inhibition was observed for the extracts obtained from bulbs of H. tubispathus and bulbs and aerial parts of H. jamesonii. The strongest cholinesterase inhibition was observed for the alkaloid extract obtained from the aerial parts for H. jamesonii (AChE IC50 = 0.7 microg/mL; BChE IC50 = 6.7 microg/mL). The AChE inhibition observed for H. jamesonii could be explained by the presence of galanthamine and sanguinine, two potent AChE inhibitors. The levels of lycorine and hippeastidine, moderate AChE inhibitors, observed in the bulbs of H. tubispathus could be responsible for the significant AChE inhibition observed. The alkaloids present in these Amaryllidaceae were identified by means of GC-MS analysis. In the case of H. tubispathus, hippeastidine and 3-O-demethylhippeastidine, were isolated and completely characterized by 1H and 13C NMR spectroscopy.
J Chromatogr B Analyt Technol Biomed Life Sci . 2007 Jun 15;853(1-2):265-74.
High-performance liquid chromatographic method with UV photodiode-array, fluorescence and mass spectrometric detection for simultaneous determination of galantamine and its phase I metabolites in biological samples[Pubmed:
17416214]
Galantamine, an alkaloid isolated from the bulbs and flowers of Caucasian snowdrop (Galanthus woronowii, Amaryllidaceae) and related species, is employed in human medicine for the treatment of various neuromuscular and neurodegenerative diseases. After the administration, the products of oxidative biotransformation (O-desmethyl-galantamine, N-desmethyl-galantamine, galantamine-N-oxide) and chiral conversion (epigalantamine) are formed in various concentrations from parent compound. For the identification and determination of galantamine and its phase I metabolites in blood plasma and tissues, a new bioanalytical method based on a reversed-phase high-performance liquid chromatography with UV photodiode-array, fluorescence and mass spectrometric detection was developed, validated and applied to pharmacokinetic and biotransformation studies. Sample preparation included a homogenization of the rat tissues (liver, brain, hypophysis) in a phosphate buffer 0.05 mol/L pH 7.4. Plasma samples and tissue homogenates were purified using a mixed-mode solid-phase extraction (Waters Oasis MCX cartridges). Galantamine, its above-mentioned metabolites and the internal standard codeine were separated on a Discovery HS F5 column (Supelco, 150 mmx4.6 mm I.D., 5 microm) at flow rate of 1 mL/min using a linear gradient elution. UV photodiode-array and mass spectrometric detection were employed for the identification of individual galantamine metabolites in various biomatrices, the fluorescence detection (lambdaexcit=280 nm/lambdaemiss=310 nm) was chosen for the quantification of galantamine and its metabolites. The developed method was applicable in liver tissue in the range from 0.50 to 63.47 nmol/g of galantamine, from 0.32 to 41.42 nmol/g of O-desmethyl-galantamine, from 0.54 to 69.40 nmol/g of N-desmethyl-galantamine and from 0.70 to 89.03 nmol/g of epigalantamine. Limit of detection was found to be 0.04 nmol/g for galantamine, 0.19 nmol/g for O-desmethyl-galantamine, and 0.07 nmol/g for N-desmethyl-galantamine and epigalantamine.