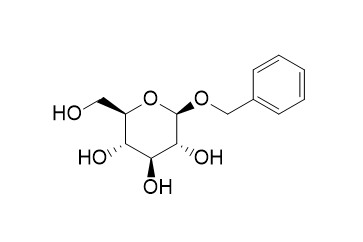
Benzyl beta-D-glucopyranoside
Benzyl β-D-glucopyranoside exhibits strong PTP1B enzyme inhibition (IC50 = 6.97) and antioxidant activity.Benzyl beta-D-glucopyranoside contribute to relieving the tension in M-rats caused by ether stress.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Curr Issues Mol Biol.2023, 45(2):1587-1600.
Indian J Pharm Sci.2022, 84(4): 874-882.
Forensic Sci Int.2022, 341:111475.
Appl. Sci.2020, 10(4),1304
Sci Rep.2024, 14(1):28864.
J Nat Prod.2019, 82(4):1002-1008
Molecules.2021, 26(19):6032.
J Inflamm Res.2022, 15:5347-5359.
Heliyon.2023, 9(11):e21944.
Am J Chin Med.2015, 30:1-22
Related and Featured Products
Nat Prod Res . 2021 Apr;35(8):1388-1392.
Phenolic glycosides from Nitraria sibirica leaves and their in vitro biological activities[Pubmed:
31379199]
Seventeen phenolic glycosides were isolated from the Nitraria sibirica. Their structures were identified by the spectroscopic data and comparison with literatures as isovanillyl alcohol-7-O-β-d-glucopyranoside (1), benzyl β-primeveroside (2), benzyl-O-β-d-glucopyranoside (3), 1-O-β-d-glucopyranosyl-4-(8-hydroxyethyl)-2-methoxyphenyl (4), dehydrosyringin (5), trans-ferulic acid-4-O-β-d-glucoside (6), cis-ferulic acid 4-O-β-d-glucopyranoside (7), glucosyringic acid (8), 1-O-feruloyl-β-d-glucoside (9), sachaloside VII (10), (3S, 5R, 6R, 7E, 9S)-megastigmane-7-ene-3-hydroxy-5,6-epoxy-9-O-β-d-glucopyranoside(11), 3-hydroxy-4,5-dimethoxybenzyl alcohol (12), pinoresinol 4-O-β-d-glucopyranoside (13), eucommin A (14), isoeucommin A (15), acanthoside (16), liriodendrin (17). All these compounds except compound 13 were isolated from the Nitraria genus for the first time. In bioactivity assays for all compounds, the compounds 8 and 15 were exhibited strong antioxidant activity (IC50 = 18.11 and 16.29 μM respectively), while compounds 3 and 11 were exhibited strong PTP1B enzymatic inhibition (IC50 = 6.97 and 11.76 μM, respectively). Furthermore, the compounds 10 and 17 were presented strong inhibitory capacities against Candida albicans (14.5 and 13.5 mm, respectively).
Carbohydr Res . 2008 Nov 24;343(17):2939-2946.
Engineering of glucoside acceptors for the regioselective synthesis of beta-(1-->3)-disaccharides with glycosynthases[Pubmed:
18828996]
Glycosynthase mutants obtained from Thermotogamaritima were able to catalyze the regioselective synthesis of aryl beta-D-Galp-(1-->3)-beta-D-Glcp and aryl beta-D-Glcp-(1-->3)-beta-D-Glcp in high yields (up to 90 %) using aryl beta-D-glucosides as acceptors. The need for an aglyconic aryl group was rationalized by molecular modeling calculations, which have emphasized a high stabilizing interaction of this group by stacking with W312 of the enzyme. Unfortunately, the deprotection of the aromatic group of the disaccharides was not possible without partial hydrolysis of the glycosidic bond. The replacement of aryl groups by benzyl ones could offer the opportunity to deprotect the anomeric position under very mild conditions. Assuming that benzyl acceptors could preserve the stabilizing stacking, benzyl beta-d-glucoside firstly assayed as acceptor resulted in both poor yields and poor regioselectivity. Thus, we decided to undertake molecular modeling calculations in order to design which suitable substituted benzyl acceptors could be used. This study resulted in the choice of 2-biphenylmethyl beta-D-glucopyranoside. This choice was validated experimentally, since the corresponding beta-(1-->3) disaccharide was obtained in good yields and with a high regioselectivity. At the same time, we have shown that phenyl 1-thio-beta-D-glucopyranoside was also an excellent substrate leading to similar results as those obtained with the O-phenyl analogue. The NBS deprotection of the S-phenyl group afforded the corresponding disaccharide quantitatively.
Carbohydr Res . 1989 Dec 1;194:185-198.
Selective bromoacetylation of alkyl hexopyranosides: a facile preparation of intermediates for the synthesis of (1----6)-linked oligosaccharides[Pubmed:
2620299]
Bromoacetylation of methyl beta-D-galacto- (1), alpha-D-galacto- (6), beta-D-gluco- (18), (22), and alpha-D-manno-pyranoside (31), and Benzyl beta-D-glucopyranoside (27), gave the corresponding 6-O-bromoacetyl derivatives 2, 7, 19, 23, 32, and 28 in 50-60% yields. Bromoacetylation of methyl 3-O-benzyl-beta-D-galactopyranoside (11) afforded methyl 3-O-benzyl-6-O-bromoacetyl-beta-D-galactopyranoside (12, 60%) as well as methyl 3-O-benzyl-2,6-di-O-bromoacetyl-beta-D-galactopyranoside (13, 14%). Compounds 2, 7, 19, 23, 32, 28, and 12 were benzoylated and the fully protected derivatives obtained were dehaloacetylated with thiourea to afford the methyl 2,3,4-tri-O-benzoyl-D-glycopyranosides of beta-galactose (5), alpha-galactose (9), beta-glucose (21), alpha-glucose (25), and alpha-mannose (34), as well as benzyl 2,3,4-tri-O-benzoyl-beta-D-glucopyranoside (30) and methyl 3-O-benzyl-2,4-di-O-benzoyl-beta-D-galactopyranoside (15). These compounds can be used as nucleophiles for the synthesis of (1----6)-linked oligosaccharides. The conversion 1----5 could be performed without isolation of the intermediates. The treatment of bromoacetyl derivatives with benzoyl chloride in pyridine resulted in the benzoylation of the remaining free hydroxyl groups and the simultaneous substitution of bromine by chlorine, yielding the corresponding mono-O-chloroacetyl derivatives. Benzoylations with benzoyl bromide avoided this secondary event. Glycosyl donors differentially substituted to allow further extension of the oligosaccharide chain at position 6 of D-glucose, D-galactose, and D-mannose, and sequentially at positions 6 and 3 in the case of the D-galactosyl donor derived from 15, were readily obtained by treatment of the appropriate, fully protected methyl glycosides with 1,1-dichloromethyl methyl ether in the presence of a catalytic amount of zinc chloride.
Bioorg Med Chem Lett . 2014 Sep 1;24(17):4120-4124.
A new phenylpropanoid and an alkylglycoside from Piper retrofractum leaves with their antioxidant and α-glucosidase inhibitory activity[Pubmed:
25127165]
Two new compounds, piperoside (1) and isoheptanol 2(S)-O-β-D-xylopyranosyl (1→6)-O-β-D-glucopyranoside (11), along with 10 known compounds 3,4-dihydroxyallylbenzene (2), 1,2-di-O-β-D-glucopyranosyl-4-allylbenzene (3), tachioside (4), benzyl-O-β-D-glucopyranoside (5), icariside F2 (6), dihydrovomifoliol-3'-O-β-D-glucopyranoside (7), isopropyl O-β-D-glucopyranoside (8), isopropyl primeveroside (9), n-butyl O-β-D-glucopyranoside (10), isoheptanol 2(S)-O-β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside (12), were isolated from the leaves of Piper retrofractum. Their structures were determined from 1D-NMR, 2D-NMR, and HR-ESI-MS spectral, a modified Mosher's method, and comparisons with previous reports. All of the isolated compounds showed modest α-glucosidase inhibitory (4.60±1.74% to 11.97±3.30%) and antioxidant activities under the tested conditions.
Biol Pharm Bull . 2004 Jan;27(1):136-137.
Effects of benzyl glucoside and chlorogenic acid from Prunus mume on adrenocorticotropic hormone (ACTH) and catecholamine levels in plasma of experimental menopausal model rats[Pubmed:
14709918]
To investigate the effectiveness of Benzyl beta-D-glucopyranoside (BG) and chlorogenic acid (CA), the constituents of the fruit of Prunus mume, for relieving tension in experimental menopausal model rats (M-rats) caused by ether stress, the effects of BG and CA on adrenocorticotropic hormone (ACTH) and catecholamine (adrenaline, noradrenaline, and dopamine) levels were examined in the plasma of M-rats. Caffeic acid, quinic acid, and rosmarinic acid, which are compounds structurally related to CA, were also examined. BG obviously recovered catecholamine levels decreased by ether stress and increased dopamine to high levels. On the other hand, CA significantly decreased the ACTH level increased by ether stress and showed the greatest effect of all compounds. These results suggest that BG and CA may contribute to relieving the tension in M-rats caused by ether stress.
Molecules . 2021 Sep 26;26(19):5827.
Isolation, Characterization, Complete Structural Assignment, and Anticancer Activities of the Methoxylated Flavonoids from Rhamnus disperma Roots[Pubmed:
34641372]
Different chromatographic methods including reversed-phase HPLC led to the isolation and purification of three O-methylated flavonoids; 5,4'-dihydroxy-3,6,7-tri-O-methyl flavone (penduletin) (1), 5,3'-dihydroxy-3,6,7,4',5'-penta-O-methyl flavone (2), and 5-hydroxy-3,6,7,3',4',5'-hexa-O-methyl flavone (3) from Rhamnus disperma roots. Additionlly, four flavonoid glycosides; kampferol 7-O-α-L-rhamnopyranoside (4), isorhamnetin-3-O-β-D-glucopyranoside (5), quercetin 7-O-α-L-rhamnopyranoside (6), and kampferol 3, 7-di-O-α-L-rhamnopyranoside (7) along with benzyl-O-β-D-glucopyranoside (8) were successfully isolated. Complete structure characterization of these compounds was assigned based on NMR spectroscopic data, MS analyses, and comparison with the literature. The O-methyl protons and carbons of the three O-methylated flavonoids (1-3) were unambiguously assigned based on 2D NMR data. The occurrence of compounds 1, 4, 5, and 8 in Rhamnus disperma is was reported here for the first time. Compound 3 was acetylated at 5-OH position to give 5-O-acetyl-3,6,7,3',4',5'-hexa-O-methyl flavone (9). Compound 1 exhibited the highest cytotoxic activity against MCF 7, A2780, and HT29 cancer cell lines with IC50 values at 2.17 μM, 0.53 μM, and 2.16 μM, respectively, and was 2-9 folds more selective against tested cancer cell lines compared to the normal human fetal lung fibroblasts (MRC5). It also doubled MCF 7 apoptotic populations and caused G1 cell cycle arrest. The acetylated compound 9 exhibited cytotoxic activity against MCF 7 and HT29 cancer cell lines with IC50 values at 2.19 μM and 3.18 μM, respectively, and was 6-8 folds more cytotoxic to tested cancer cell lines compared to the MRC5 cells.
Phytochemistry . 2011 Jun;72(8):791-795.
Unusual glycosides of pyrrole alkaloid and 4'-hydroxyphenylethanamide from leaves of Moringa oleifera[Pubmed:
21439596]
Glycosides of pyrrole alkaloid (pyrrolemarumine 4″-O-α-L-rhamnopyranoside) and 4'-hydroxyphenylethanamide (marumosides A and B) were isolated from leaves of Moringa oleifera along with eight known compounds; niazirin, methyl 4-(α-L-rhamnopyranosyloxy)benzylcarbamate, benzyl β-D-glucopyranoside, benzyl β-D-xylopyranosyl-(1→6)-β-D-glucopyranoside, kaempferol 3-O-β-D-glucopyranoside, quercetin 3-O-β-D-glucopyranoside, adenosine and L-tryptophan. Structure elucidations were based on analyses of chemical and spectroscopic data including 1D- and 2D-NMR.
Phytochem Anal . 2013 Feb;24(2):129-134.
Purification of Phenylalkanoids and monoterpene glycosides from Rhodiola rosea L. roots by high-speed counter-current chromatography[Pubmed:
22811209]
Introduction: Rhodiola rosea L. is a medicinal herb used for its adaptogenic properties. The main active components are the phenylpropanoids collectively referred to as rosavins.
Objectives: To develop an isolation method for phytochemicals present in Rhodiola rosea roots using high-speed counter-current chromatography (HSCCC).
Methodology: The roots of Rhodiola rosea were extracted with methanol and fractionated using liquid-liquid partition and polyamide column clean-up. The purified fraction (100 mg) was subjected to semi-preparative HSCCC using the two-phase solvent system ethyl acetate:butanol:water (3:2:5). The head-to-tail elution mode was employed with a flow rate of 1.5 mL/min and a rotary speed of 1000 rpm.
Results: The separation yielded six main fractions with four components more than 90% pure. The sixth fraction was further purified using semi-preparative HPLC with a Synergi-hydro RP C₁₈ -column to obtain rosin and geranyl 1-O-α-l-arabinopyranosyl(1 → 6)-β-d-glucopyranoside. The main components isolated were rosavin (3.4 mg, 97% purity), salidroside (0.5 mg, 90% purity), benzyl-O-β-d-glucopyranoside (1.2 mg, 85% purity), rosarin (1.3 mg, 99% purity), rosiridin (1.8 mg, 92% purity), rosin (1.2 mg, 95% purity) and geranyl 1-O-α-l-arabinopyranosyl(1 → 6)-β-d-glucopyranoside (6.5 mg, 97% purity). The identity and purity of these components were confirmed using ultrafast liquid chromatography-diode-array detector-MS/MS analysis, 1H- and 13C-NMR spectroscopy.
Conclusion: High-speed counter-current chromatography was successful in the isolation of several phytochemicals present in Rhodiola rosea roots, including two components that are not commercially available.
Molecules . 2021 Sep 26;26(19):5827.
Isolation, Characterization, Complete Structural Assignment, and Anticancer Activities of the Methoxylated Flavonoids from Rhamnus disperma Roots[Pubmed:
34641372]
Different chromatographic methods including reversed-phase HPLC led to the isolation and purification of three O-methylated flavonoids; 5,4'-dihydroxy-3,6,7-tri-O-methyl flavone (penduletin) (1), 5,3'-dihydroxy-3,6,7,4',5'-penta-O-methyl flavone (2), and 5-hydroxy-3,6,7,3',4',5'-hexa-O-methyl flavone (3) from Rhamnus disperma roots. Additionlly, four flavonoid glycosides; kampferol 7-O-α-L-rhamnopyranoside (4), isorhamnetin-3-O-β-D-glucopyranoside (5), quercetin 7-O-α-L-rhamnopyranoside (6), and kampferol 3, 7-di-O-α-L-rhamnopyranoside (7) along with benzyl-O-β-D-glucopyranoside (8) were successfully isolated. Complete structure characterization of these compounds was assigned based on NMR spectroscopic data, MS analyses, and comparison with the literature. The O-methyl protons and carbons of the three O-methylated flavonoids (1-3) were unambiguously assigned based on 2D NMR data. The occurrence of compounds 1, 4, 5, and 8 in Rhamnus disperma is was reported here for the first time. Compound 3 was acetylated at 5-OH position to give 5-O-acetyl-3,6,7,3',4',5'-hexa-O-methyl flavone (9). Compound 1 exhibited the highest cytotoxic activity against MCF 7, A2780, and HT29 cancer cell lines with IC50 values at 2.17 μM, 0.53 μM, and 2.16 μM, respectively, and was 2-9 folds more selective against tested cancer cell lines compared to the normal human fetal lung fibroblasts (MRC5). It also doubled MCF 7 apoptotic populations and caused G1 cell cycle arrest. The acetylated compound 9 exhibited cytotoxic activity against MCF 7 and HT29 cancer cell lines with IC50 values at 2.19 μM and 3.18 μM, respectively, and was 6-8 folds more cytotoxic to tested cancer cell lines compared to the MRC5 cells.