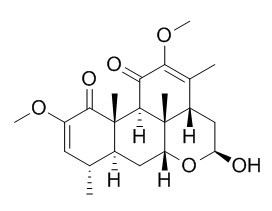
Neoquassine
Quassin, neoquassin and picrasinoside B are insecticide quassinoids.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Proc Natl Acad Sci USA.2016, 113(30):E4407-1
J Applied Biological Chemistry2021, 64(2):185-192
J Korean Med Ophthalmol Otolaryngol Dermatol2023, 36(1):21-39.
Food Bioscience2023, 59:103903
Pamukkale Medical Journal2022, 15(4):796-803.
Int J Mol Sci.2021, 22(8):4211.
Drug Test Anal.2018, 10(10):1579-1589
Vietnam Journal of Food Control2022, 5(3):pp.390-401.
Antioxidants (Basel).2021, 10(10):1620.
Molecules.2024, 29(5):1171.
Related and Featured Products
J. Agric. Food Chem.,2010, 58(5):2807-11.
Liquid chromatography electrospray ionization tandem mass spectrometric determination of quassin and neoquassin in fruits and vegetables.[Pubmed:
20196620]
Quassia amara wood chips are used by organic farmers as a valid alternative to synthetic insecticides. The powder of Q. amara contains high levels of quassin, neoquassin and picrasinoside B. In this study we developed a liquid chromatography mass spectrometry method for the rapid and accurate quantification of the insecticide quassinoids on fruits and vegetables.
METHODS AND RESULTS:
Quassinoids were extracted from fruits and vegetables with acetonitrile and separated on a Zorbax Column Eclipse XDB C8 by isocratic elution with a mobile phase consisting of water and methanol with 0.1% of formic acid. Using a high-performance liquid chromatograph coupled with an electrospray ionization tandem mass spectrometer (HPLC/ESI-MS/MS), quassinoids were selectively and simultaneously detected monitoring the multiple reaction (MRM) transitions of proton adduct precursor ions: m/z 389.5 --> 222.9 for quassin, 391.5 --> 372.9 for neoquassin and 576.1 --> 394.5 for picrasinoside B. For all quassinoids calibration was linear over a working range of 1 and 100 microg/kg with r > 0.991. Limit of determination (LOD) and limit of quantification (LOQ) for both quassinoids were 0.5 and 1 microg/kg respectively while for picrasinoside B they were 5 and 10 microg/kg. Quassinoid recoveries ranged from 85.3% to 105.3% with coefficients of variation between 2.5% and 12.8% for fruit and vegetables. The presence of interfering compounds in the fruit and vegetable extract was evaluated and found to be minimal.
CONCLUSIONS:
Due to the linear behavior it was concluded that the multiple reaction transitions of precursor ions can be used for analytical purposes, i.e. for identification and quantification of quassin, neoquassin(Neoquassine), and picrasinoside B in fruit and vegetable extracts at trace levels.