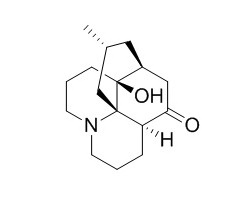
Lycodoline
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
LWT - Food Science and Technology2022, 164:113627
Int J Mol Med.2019, 43(6):2516-2522
Biomol Ther (Seoul).2023, 31(1):40-47.
Bull. Pharm. Sci., Assiut University2020, 43(2):149-155.
J Chromatogr A.2024, 1714:464544.
J Pharm Biomed Anal.2016, 129:50-59
Exp Biol Med (Maywood).2019, 244(16):1463-1474
Drug Des Devel Ther.2023, 17:2461-2479.
Sci Rep.2018, 8:15059
Synthetic and Systems Biotechnology2023, j.synbio.
Related and Featured Products
Phytochemistry. 2010 Feb;71(2-3):149-57.
Acetylcholinesterase inhibitory activity of lycopodane-type alkaloids from the Icelandic Lycopodium annotinum ssp. alpestre.[Pubmed:
19939421]
The aim of this study was to investigate structures and acetylcholinesterase inhibitory activities of lycopodane-type alkaloids isolated from an Icelandic collection of Lycopodium annotinum ssp. alpestre. Ten alkaloids were isolated, including annotinine, annotine, Lycodoline, lycoposerramine M, anhydroLycodoline, gnidioidine, lycofoline, lannotinidine D, and acrifoline, as well as a previously unknown N-oxide of annotine.
METHODS AND RESULTS:
1H and 13C NMR data of several of the alkaloids were provided for the first time. Solvent-dependent equilibrium constants between ketone and hemiketal form of acrifoline were determined. Conformation of acrifoline was characterized using NOESY spectroscopy and molecular modelling. The isolated alkaloids were evaluated for their in vitro inhibitory activity against acetylcholinesterase and butyrylcholinesterase. Ligand docking studies based on mutated 3D structure of Torpedo californica acetylcholinesterase provided rationale for low inhibitory activity of the isolated alkaloids as compared to huperzine A or B, which are potent acetylcholinesterase inhibitors belonging to the lycodine class. Based on the modelling studies the lycopodane-type alkaloids seem to fit well into the active site gorge of the enzyme but the position of their functional groups is not compatible with establishing strong hydrogen bonding interactions with the amino acid residues that line the binding site.
CONCLUSIONS:
The docking studies indicate possibilities of additional functionalization of the lycopodane skeleton to render potentially more active analogues.
Zhongguo Zhong Yao Za Zhi. 2012 Feb;37(4):475-7.
Study on chemical constituents of Lycopodium alkaloids[Pubmed:
22667147]
To study the alkaloid chemical constituents of Lycopodium japonicum.
METHODS AND RESULTS:
Compounds were isolated and purified by such methods as silica gel column chromatography, RP-C18 reversed phase column chromatography, Sephadex LH-20 column chromatography and Waters semi-preparative liquid chromatogram, and their structures were identified based on physicochemical property and spectrum data. Nine known alkaloid chemical constituents were isolated and identified, they were Lycodoline (1), lucidioline (2), alpha-obscurine (3), lycopodine (4), lycoposerramine-L (5), lycoposerramine-M (6), 11alpha-O-acetyl-lycopodine (7), des-N-methyl-a-obscurine (8), clavolonine (9).
CONCLUSIONS:
Compounds 4-9 were obtained from L. japonicum for the first time.