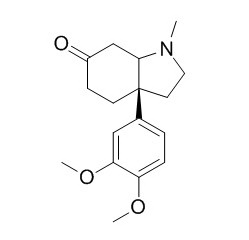
Mesembrine
Mesembrine is an inhibitor of phosphodiesterase-4 (PDE4).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Neurobiol.2022, 02873-9.
Biomedicines.2021, 9(8):954.
J Mol Recognit.2020, 33(2):e2819
Am J Chin Med.2015, 30:1-22
Fitoterapia.2024, 177:106138.
Food Chem Toxicol.2024, 186:114589.
Food Bioscience2023, 52:102412
Environ Toxicol.2020, doi: 10.1002
Curr Issues Mol Biol.2022, 44(5):2300-2308.
Internoational J of Toxicology2020, 10.1177.
Related and Featured Products
Planta Med. 2012 Feb;78(3):260-8.
In vitro permeation of mesembrine alkaloids from Sceletium tortuosum across porcine buccal, sublingual, and intestinal mucosa.[Pubmed:
22105579]
Sceletium tortuosum is an indigenous South African plant that has traditionally been used for its mood-enhancing properties. Recently, products containing S. tortuosum have become increasingly popular and are commonly administered as tablets, capsules, teas, decoctions, or tinctures, while traditionally the dried plant material has been masticated.
METHODS AND RESULTS:
This study evaluated the in vitro permeability of the four major S. tortuosum alkaloids (i.e., Mesembrine, mesembrenone, mesembrenol, and mesembranol) across porcine intestinal, sublingual, and buccal tissues in their pure form and in the form of three different crude plant extracts, namely water, methanol, and an acid-base alkaloid-enriched extract. The permeability of Mesembrine across intestinal tissue was higher than that of the highly permeable reference compound caffeine (which served as a positive control for membrane permeability) both in its pure form, as well as in the form of crude extracts. The intestinal permeability of mesembranol was similar to that of caffeine, while those of mesembrenol and mesembrenone were lower than that of caffeine, but much higher than that of the poorly permeable reference compound atenolol (which served as a negative control for membrane permeability). In general, the permeabilities of the alkaloids were lower across the sublingual and the buccal tissues than across the intestinal tissue. However, comparing the transport of the alkaloids with that of the reference compounds, there are indications that transport across the membranes of the oral cavity may contribute considerably to the overall bioavailability of the alkaloids, depending on pre-systemic metabolism, when the plant material is chewed and kept in the mouth for prolonged periods.
CONCLUSIONS:
The results from this study confirmed the ability of the alkaloids of S. tortuosum in purified or crude extract form to permeate across intestinal, buccal, and sublingual mucosal tissues.
Org Lett. 2009 Feb 5;11(3):555-8.
Palladium-catalyzed sequential arylation and allylic alkylation of highly functionalized ketones: a concise synthesis of mesembrine.[Pubmed:
19117400]
METHODS AND RESULTS:
An unprecedented palladium-catalyzed sequential procedure toward arylation and allylic alkylation of highly functionalized cyclohexenones was developed. This new protocol leads to useful building blocks containing a benzylic quaternary carbon in only one step.
CONCLUSIONS:
A concise total synthesis of Mesembrine based on this procedure was achieved in only five steps with 22% overall yield.
J Org Chem. 2013 Apr 5;78(7):3410-5.
Double reduction of cyclic aromatic sulfonamides: synthesis of (+)-mesembrine and (+)-mesembranol.[Pubmed:
23496752]
METHODS AND RESULTS:
The synthesis of (+)-Mesembrine (1) and (+)-mesembranol (2) has been achieved from the monoterpene (S)-(-)-perillyl alcohol. Key transformations include a diastereo- and regioselective Pd-mediated intramolecular Heck reaction, and a double reduction of the resultant cyclic sulfonamide, to afford the cis-3a-aryloctahydroindole skeleton.