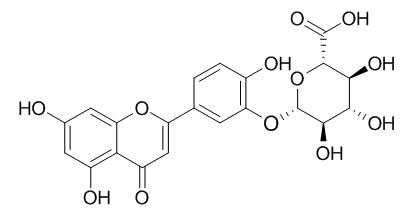
Luteolin-3-O-beta-D-glucuronide
Luteolin 3'-O-beta-D-glucuronide is active in the inhibition of nitrite production in macrophages.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Asian Nat Prod Res.2019, 5:1-17
Psychopharmacology (Berl).2020, 10.1007
PLoS One.2021, 16(6):e0248479.
Curr Issues Mol Biol.2024, 46(6):6018-6040.
Phytomedicine2022, 104:154337.
Evid Based Complement Alternat Med.2016, 2016:1739760
Agronomy 2021, 11(3),502.
Inflammation.2024, 02034-1.
Biomed Pharmacother.2024, 179:117346.
Comparative Clinical Pathology 2021, 30:961-971.
Related and Featured Products
J Agric Food Chem. 2004 Aug 11;52(16):4987-92.
Flavonoid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. postulation of a biosynthetic pathway.[Pubmed:
15291464 ]
The distribution of seven flavonoids, eriocitrin, luteolin 3'-O-beta-d-glucuronide(Luteolin-3-O-beta-D-glucuronide), hesperidin, diosmin, isoscutellarein 7-O-glucoside, hispidulin 7-O-glucoside, and genkwanin, has been studied in Rosmarinus officinalis leaves, flowers, stems, and roots during plant growth.
METHODS AND RESULTS:
The maximum level reached by luteolin 3'-O-beta-d-glucuronide(Luteolin-3-O-beta-D-glucuronide) in leaves during June-August suggests the existence of a delay between the activation of the enzymes involved in the flavanone and flavone biosynthesis. The presence of hesperidin and diosmin in the vascular system is significant, and hesperidin shows even higher levels than the phenolic diterpenes and rosmarinic acid.
CONCLUSIONS:
The distribution of flavonoids observed in R. officinalis suggests a functional and structural relationship between phytoregulators and flavonoids, where flavonoids would be "protectors" of the activity of phytoregulators. A hypothesis for the general pathway of biosynthesis of these compounds in plants of the family Labiatae is proposed.
J Agric Food Chem. 2010 May 12;58(9):5363-7.
Flavonoids and phenolic compounds from Rosmarinus officinalis.[Pubmed:
20397728 ]
A new flavonoid, 6''-O-(E)-feruloylhomoplantaginin (1), and 14 known compounds, 6''-O-(E)-feruloylnepitrin (2), 6''-O-(E)-p-coumaroylnepitrin (3), 6-methoxyluteolin 7-glucopyranoside (4), luteolin 3'-O-beta-D-glucuronide (Luteolin-3-O-beta-D-glucuronide,5), luteolin 3'-O-(3''-O-acetyl)-beta-D-glucuronide (6), kaempferol (7), luteolin (8), genkwanin (9), and ladanein (10), together with 1-O-feruloyl-beta-D-glucopyranose (11), 1-O-(4-hydroxybenzoyl)-beta-D-glucopyranose (12), rosmarinic acid (13), carnosic acid (14), and carnosol (15), were isolated from the leaves of Rosmarinus officinalis .
METHODS AND RESULTS:
The structures were established on the basis of NMR spectroscopic methods supported by HRMS. All isolated compounds were tested for cytotoxicity in human cancer cell lines (HepG2, COLO 205, and HL-60) and for anti-inflammatory activities in lipopolysaccharide (LPS)-treated RAW 264.7 macrophage cells. Among them, compounds 14 and 15 were modestly active in the inhibition of nitrite production in macrophages, followed by compounds 8 and 5. Compounds 14 and 15 were more effective as an antiproliferative agent in HL-60 cells with IC(50) values of 1.7 and 5.5 microM, followed by compounds 8 and 7 with IC(50) of 39.6 and 82.0 microM, respectively. In addition, compounds 14 and 15 showed potent antiproliferative effects on COLO 205 cells with IC(50) values of 32.8 and 29.9 microM, respectively.