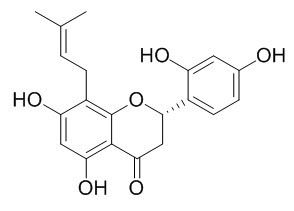
Leachianone G
Leachianone G exhibits significant antioxidant potentials in the ABTS, ONOO(-), and total ROS assays, it also shows inhibitory activities against pancreatic lipase, it may be used as drug candidate to control obesity. Leachianone G shows potent antiviral activity (IC(50) = 1.6 microg/ml). (+/-)Leachianone G 1b, bearing 8-prenyl and 2',4'-dihydoxyl groups, exhibits potent vasorelaxant and neuroprotective effects.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nat Prod Sci.2018, 24(2):109-114
Heliyon.2022, 8(12):e12031.
LWT2024, 200:116184.
Pharmaceuticals (Basel).2024, 17(8):1001.
J Ginseng Res.2023, 47(4):572-582.
Hum. Ecol. Res.2025, 63(2):165-174
Antioxidants (Basel).2019, 8(8):E307
J Pharm Biomed Anal.2022, 207:114398.
Appl Biochem Biotechnol.2020, 190(2):732-744
Antioxidants (Basel).2020, 9(4):284.
Related and Featured Products
Phytochemistry. 2003 Apr;62(7):1093-9.
Efficient production and capture of 8-prenylnaringenin and leachianone G-biosynthetic intermediates of sophoraflavanone G--by the addition of cork tissue to cell suspension cultures of Sophora flavescens.[Pubmed:
12591262]
It has previously been demonstrated that the addition of cork tissue to cell suspension cultures of Sophora flavescens stimulates the production of sophoraflavanone G, most of which has been recovered from the added cork tissue.
METHODS AND RESULTS:
In the present study, it was found that two precursors of sophoraflavanone G, 8-prenylnaringenin (sophoraflavanone B) and Leachianone G, both of which have never been detected either in cultured cells or in the original plants, also accumulated in the added cork tissue. Thirteen minor flavonoids including three prenylated flavonoids, in addition to 8-prenylnaringenin and Leachianone G, were isolated from the cork tissue co-incubated with S. flavescens cells.
CONCLUSIONS:
The new compounds flavescenones A, B and C, were determined to be (3R)-5, 7, 2'-trihydroxy-6-gamma, gamma-dimethylallyl-4', 5'-methylenedioxyisoflavanone; 5, 7, 2'-trihydroxy-6-gamma, gamma-dimethylallyl-4', 5'-methylenedioxyisoflavone and 2-[2',4'-dihydroxy-3'-(gamma-hydroxymethyl-gamma-methylallyl)phenyl]-5,6-methylenedioxybenzofuran, respectively, by means of spectroscopic analyses that included 2D-NMR techniques.
Nat Prod Res. 2017 Apr 26:1-7.
Obesity is the worst health risk worldwide, which is linked to a number of diseases. Pancreatic lipase is considered as an affective cause of obesity and can be a major target for controlling the obesity. The present study was designed to find out best ph[Pubmed:
28446025]
Obesity is the worst health risk worldwide, which is linked to a number of diseases. Pancreatic lipase is considered as an affective cause of obesity and can be a major target for controlling the obesity. The present study was designed to find out best phytochemicals against pancreatic lipase through molecular docking combined with molecular dynamics (MD) simulation.
METHODS AND RESULTS:
For this purpose, a total of 3770 phytochemicals were docked against pancreatic lipase and ranked them on the basis of binding affinity. Finally, 10 molecules (Kushenol K, Rosmarinic acid, Reserpic acid, Munjistin, Leachianone G, Cephamycin C, Arctigenin, 3-O-acetylpadmatin, Geniposide and Obtusin) were selected that showed strong bonding with the pancreatic lipase. MD simulations were performed on top five compounds using AMBER16. The simulated complexes revealed stability and ligands remained inside the binding pocket.
CONCLUSIONS:
This study concluded that these finalised molecules can be used as drug candidate to control obesity.
Biol Pharm Bull. 2008 May;31(5):908-15.
Re-evaluation of the antioxidant prenylated flavonoids from the roots of Sophora flavescens.[Pubmed:
18451517]
This was in contrast to the prenylated flavanones, sophoraflavanone G and kurarinone, which were isolated from the methylene chloride (CH(2)Cl(2)) fraction of the same source.
METHODS AND RESULTS:
Five flavanones consisting of kushenol E, Leachianone G, kurarinol, sophoraflavanone G, and kurarinone exhibited significant antioxidant potentials in the ABTS, ONOO(-), and total ROS assays; however, the prenylated chalcones and prenylated flavonol showed more potent scavenging/inhibitory activities than the prenylated flavanones. Therefore, the prenylated chalcones and prenylated flavonol, rather than the prenylated flavanones, may make important contributions toward the marked antioxidant capacities of S. flavescens. Furthermore, kuraridinol, kurarinol, and kurarinone showed significant inhibitory activities against intracellular ROS levels as well as NF-kappaB activation by t-BHP.
CONCLUSIONS:
Overall, the results indicate that S. flavescens and its prenylated flavonoids may possess good anti-inflammatory activity, which is implicated in their significant antioxidant activity.
Phytochemistry. 2003 Apr;62(8):1235-8.
Antiviral flavonoids from the root bark of Morus alba L.[Pubmed:
12648543]
METHODS AND RESULTS:
A prenylated flavonoid, moralbanone, along with seven known compounds kuwanon S, mulberroside C, cyclomorusin, eudraflavone B hydroperoxide, oxydihydromorusin, Leachianone G and alpha-acetyl-amyrin were isolated from the root bark of Morus alba L.
CONCLUSIONS:
Leachianone G showed potent antiviral activity (IC(50) = 1.6 microg/ml), whereas mulberroside C showed weak activity (IC(50) = 75.4 microg/ml) against herpes simplex type 1 virus (HSV-1). Their structures were elucidated by spectroscopic methods.
Plant Physiol. 2003 Nov;133(3):1306-13.
Characterization of leachianone G 2"-dimethylallyltransferase, a novel prenyl side-chain elongation enzyme for the formation of the lavandulyl group of sophoraflavanone G in Sophora flavescens Ait. cell suspension cultures.[Pubmed:
14551337]
METHODS AND RESULTS:
Leachianone G (LG) 2"-dimethylallyltransferase, a novel prenyl side-chain elongation enzyme, was identified in Sophora flavescens Ait. cultured cells.
The enzyme transfers a dimethylallyl group to the 2" position of another dimethylallyl group attached at position 8 of LG to form sophoraflavanone G, a branched monoterpenoid-conjugated flavanone characteristic to this plant. This membrane-bound dimethylallyltransferase required Mg2+ (optimum concentration was 10 mm) for the reaction and had an optimum pH of 8.8. It utilized dimethylallyl diphosphate as the sole prenyl donor, and the 2'-hydroxy function in LG was indispensable to the activity. The apparent Km values for dimethylallyl diphosphate and LG were 59 and 2.3 microm, respectively. Subcellular localization of three enzymes that participated in the formation of the lavandulyl group was also investigated by sucrose density gradient centrifugation. Two prenyltransferases, naringenin 8-dimethylallyltransferase and LG 2"-dimethylallyltransferase, were localized in the plastids, whereas 8-dimethylallylnaringenin 2'-hydroxylase, which catalyzes the crucial step in the lavandulyl-group formation, was associated with the endoplasmic reticulum.
CONCLUSIONS:
These results suggest the close cooperation between the plastids and the endoplasmic reticulum in the formation of lavandulyl groups.
Bioorg Med Chem Lett. 2009 Jun 15;19(12):3196-8.
Synthesis, biological evaluation of prenylflavonoids as vasorelaxant and neuroprotective agents.[Pubmed:
19442520 ]
METHODS AND RESULTS:
A series of prenylflavonoids with multiple hydroxyl groups were synthesized and evaluated for their vasorelaxant activities against rat aorta rings pre-contracted by phenylephrine (PE), as well as their neuroprotective effects against OGD induced PC12 cell injury.
CONCLUSIONS:
The results indicated that the prenyl group at A-ring of prenylflavonoids, as well as hydroxyl groups at B-ring was important for their activities. (+/-)Leachianone G 1b, bearing 8-prenyl and 2',4'-dihydoxyl groups, exhibited the most potent vasorelaxant and neuroprotective effects.