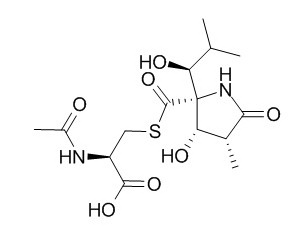
Lactacystin
Lactacystin is a selective UPS inhibitor recently used to destroy dopamine (DA) neurons in animal models of Parkinson's disease (PD); marked differences in the rotational response to apomorphine and l-DOPA suggest different mechanisms of neurodegeneration evoked by Lactacystin and 6-OHDA. Lactacystin induces cell death and α-synuclein-positive inclusions in cytoplasm, it has diversified killing effects on gastric cancer cells, the mechanism may be related to induce the apoptosis by downregulation of nuclear factor kappa B viability.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nutrients2022, 14(3),695.
Plants (Basel).2021, 10(4):702.
World J Microbiol Biotechnol.2024, 40(9):265.
Biochem Pharmacol.2017, 130:10-20
J Sep Sci.2018, 41(11):2488-2497
J.of Traditional&Complementary Med.2022, 10.1016:j.jtcme.
Chem. of Vegetable Raw Materials2020, 97-105
J Sep Sci.2020, 201901140
New Journal of Chemistry2019, 43:12538-12547
Int J Mol Sci.2023, 24(5):4505.
Related and Featured Products
Tumour Biol. 2015 May;36(5):3465-70.
Investigation the mechanism of the apoptosis induced by lactacystin in gastric cancer cells.[Pubmed:
25541208]
The study aims to investigate the relationship between nuclear factor (nuclear factor kappa B (NF-κB)) viability and Lactacystin-mediated cell apoptosis in gastric cancer cells.
METHODS AND RESULTS:
Two gastric cancer cell lines (MKN28 and SGC7901) were treated with Lactacystin-a proteasome inhibitor for 24 h. The cell viability, toxicity, and death were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. DNA binding viability of NF-κB and caspase-3 viability were analyzed by ELISA; the expression of p65 NF-κB nuclear protein was detected by immunocytochemistry and Western blot. Lactacystin reduced DNA binding viability of NF-κB (t = 3.0,P = 0.013) and the NF-κB viability (compared to the 5, 10 μmol/L MKN28 cell (p53 mutant) line, P < 0.001) and the expression of p65 NF-κB nuclear protein decreased parallelled to concentrations of Lactacystin in MKN28 cell line, while without obvious effects on NF-κB viability in SGC7901 cell line (P = 0.381), while the viability of caspase-3 increased also along with the raising of Lactacystin concentrations (compared to control, 5 μmol/L: SGC7901 cell line P = 0.029, MKN28 cell line P < 0.001; 10 μmol/L: SGC7901 cell line, P < 0.001, MKN28 cell line, P < 0.001).
CONCLUSIONS:
It was concluded that Lactacystin had diversified killing effects on gastric cancer cells. The mechanism may be related to induce the apoptosis by downregulation of nuclear factor kappa B viability. There may be additional cell survival/death pathway in SGC7901 gastric cancer cells.
Behav Brain Res. 2015 Apr 15;283:203-14.
Decreased behavioral response to intranigrally administered GABAA agonist muscimol in the lactacystin model of Parkinson's disease may result from partial lesion of nigral non-dopamine neurons: comparison to the classical neurotoxin 6-OHDA.[Pubmed:
25655509]
Lactacystin is a selective UPS inhibitor recently used to destroy dopamine (DA) neurons in animal models of Parkinson's disease (PD). However, both in vitro and in vivo studies show discrepancies in terms of the sensitivity of non-DA neurons to its toxicity.
METHODS AND RESULTS:
Therefore, our study was aimed to examine the toxic effect of intranigral administration of Lactacystin on DA and non-DA neurons in the rat substantia nigra (SN), compared to the classic neurotoxin 6-OHDA. Tissue DA levels in the striatum and SN and GABA levels in the SN were also examined. Moreover, behavioral response of nigral GABAA receptors to locally administered muscimol was evaluated in these two PD models. We found that both Lactacystin and 6-OHDA induced a strong decrease in DA level in the lesioned striatum and SN but only Lactacystin slightly reduced GABA levels in the SN. A stereological analysis showed that both neurotoxins highly decreased the number of DA neurons in the SN, while only Lactacystin moderately reduced the number of non-DA ones. Finally, in the Lactacystin group, the number of contralateral rotations after intranigrally administrated muscimol was decreased in contrast to the increased response in the 6-OHDA model.
CONCLUSIONS:
Our study proves that, although Lactacystin is not a fully selective to DA neurons, these neurons are much more vulnerable to its toxicity. Partial lesion of nigral non-DA neurons in this model may explain the decreased behavioral response to the GABAA agonist muscimol.
Br J Pharmacol. 2015 Aug;172(16):4200-15.
Neurorestoration induced by the HDAC inhibitor sodium valproate in the lactacystin model of Parkinson's is associated with histone acetylation and up-regulation of neurotrophic factors.[Pubmed:
26040297]
Histone hypoacetylation is associated with Parkinson's disease (PD), due possibly to an imbalance in the activities of enzymes responsible for histone (de)acetylation; correction of which may be neuroprotective/neurorestorative. This hypothesis was tested using the anti-epileptic drug sodium valproate, a known histone deacetylase inhibitor (HDACI), utilizing a delayed-start study design in the Lactacystin rat model of PD.
METHODS AND RESULTS:
The irreversible proteasome inhibitor Lactacystin was unilaterally injected into the substantia nigra of Sprague-Dawley rats that subsequently received valproate for 28 days starting 7 days after Lactacystin lesioning. Longitudinal motor behavioural testing, structural MRI and post-mortem assessment of nigrostriatal integrity were used to track changes in this model of PD and quantify neuroprotection/restoration. Subsequent cellular and molecular analyses were performed to elucidate the mechanisms underlying valproate's effects.
Despite producing a distinct pattern of structural re-modelling in the healthy and Lactacystin-lesioned brain, delayed-start valproate administration induced dose-dependent neuroprotection/restoration against Lactacystin neurotoxicity, characterized by motor deficit alleviation, attenuation of morphological brain changes and restoration of dopaminergic neurons in the substantia nigra. Molecular analyses revealed that valproate alleviated Lactacystin-induced histone hypoacetylation and induced up-regulation of brain neurotrophic/neuroprotective factors.
CONCLUSIONS:
The histone acetylation and up-regulation of neurotrophic/neuroprotective factors associated with valproate treatment culminate in a neuroprotective and neurorestorative phenotype in this animal model of PD. As valproate induced structural re-modelling of the brain, further research is required to determine whether valproate represents a viable candidate for disease treatment; however, the results suggest that HDACIs could hold potential as disease-modifying agents in PD.
Neurosci Lett. 2014 Jul 11;575:25-30.
Differential protein profile of PC12 cells exposed to proteasomal inhibitor lactacystin.[Pubmed:
24858133]
Parkinson's disease (PD) is the second most common neurodegenerative disease worldwide and recent studies implicate a central role for ubiquitin-proteasome system (UPS) impairment in the etiopathogenesis of PD.
METHODS AND RESULTS:
To explore the possible role of UPS dysfunction in PD and the proteins involved, PC12 cells were treated with 10μM Lactacystin, a 20S proteasome inhibitor, for 24h. Lactacystin induced cell death and α-synuclein-positive inclusions in cytoplasm. Following two-dimensional difference in-gel electrophoresis (2-D DIGE) which was used to separate the cellular proteins, the proteins that were significantly altered were analyzed and identified. Proteomic study identified 6 differentially expressed proteins between Lactacystin-treated and control cells in this study. Four proteins (heat shock 70kDa protein 8, 78kDa glucose-regulated protein, serine proteinase inhibitor clade B member 6 and aldehyde reductase) were increased and 2 proteins (peripherin and tyrosine hydroxylase) were decreased following proteasomal inhibition. The results revealed that PC12 cells treated with 10μM Lactacystin for 24h could be used as a cellular model of PD.
CONCLUSIONS:
The proteins identified in the present indicate not only the damage of proteasomal inhibition to the cells but also the possible responses of the cells. These data show that proteomic study may provide information relevant to biological basis for PD and potential new treatment targets.
Behav Brain Res. 2014 Mar 15;261:79-88.
Chronic L-DOPA treatment attenuates behavioral and biochemical deficits induced by unilateral lactacystin administration into the rat substantia nigra.[Pubmed:
24361083]
The aim of the study was to determine whether the dopamine (DA) precursor l-DOPA attenuates parkinsonian-like symptoms produced by the ubiquitin-proteasome system inhibitor Lactacystin.
METHODS AND RESULTS:
Wistar rats were injected unilaterally with Lactacystin (2.5 μg/2 μl) or 6-OHDA (8 μg/2 μl) into the substantia nigra (SN) pars compacta. Four weeks after the lesion, the animals were treated chronically with l-DOPA (25 or 50 mg/kg) for two weeks. During l-DOPA treatment, the Lactacystin-treated rats were tested for catalepsy and forelimb asymmetry. Rotational behavior was evaluated after apomorphine (0.25 mg/kg) and l-DOPA in both PD models. After completion of experiments, the animals were killed and the levels of DA and its metabolites in the striatum and SN were assayed. We found that acute l-DOPA administration effectively decreased catalepsy and increased the use of the compromised forelimb in the cylinder test. However, the Lactacystin group did not respond to apomorphine or acute l-DOPA administration in the rotational test. Repeated l-DOPA treatment produced contralateral rotations in both PD models, but the number of rotations was much greater in the 6-OHDA-lesioned rats. Both toxins markedly (>90%) reduced the levels of DA and its metabolites in the striatum and SN, while l-DOPA diminished these decreases, especially in the SN.
CONCLUSIONS:
By demonstrating the efficacy of l-DOPA in several behavioral tests, our study confirms the usefulness of the Lactacystin lesion as a model of PD. However, marked differences in the rotational response to apomorphine and l-DOPA suggest different mechanisms of neurodegeneration evoked by Lactacystin and 6-OHDA.
Quercetin 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No: CFN95088
CAS No: 143061-65-8
Price: $338/10mg
Daidzein-4',7-diglucoside
Catalog No: CFN95094
CAS No: 53681-67-7
Price: $288/10mg
Tuberosin
Catalog No: CFN95107
CAS No: 41347-45-9
Price: $218/5mg
3,7,23,24-tetrahydroxycucurbita-5,25-dien-19-al
Catalog No: CFN95168
CAS No: 1446447-97-7
Price: $318/5mg
Isonardosinone
Catalog No: CFN95180
CAS No: 27062-01-7
Price: $318/10mg
[(1(10)E,2R,4R)]-2-Methoxy-8,12-epoxygemacra-1(10),7,11-trien-6-one
Catalog No: CFN95198
CAS No: 75412-95-2
Price: $318/10mg
5,6,7,3',4',5'-Hexamethoxyflavanone
Catalog No: CFN95376
CAS No: 74064-17-8
Price: $318/10mg
Citrusin C
Catalog No: CFN95420
CAS No: 18604-50-7
Price: $318/10mg
Massonianoside D
Catalog No: CFN95500
CAS No: 85115-04-4
Price: $318/10mg
Regaloside I
Catalog No: CFN95525
CAS No: 126239-78-9
Price: $318/5mg