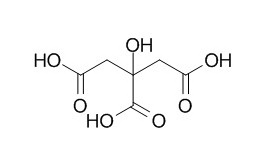
Citric acid
Citric acid is a natural preservative and food tartness enhancer, it is also an Al-chelating substance and a natural antioxidant. Citric acid (1-2 g/kg) can decrease brain lipid peroxidation and inflammation, liver damage, and DNA fragmentation. Citric acid has phytoremediation of heavy metal contaminated soil; citric acid can decrease the adsorption of both lead and cadmium. Dietary citric acid can effectively improve phytate P utilization in chicks.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem.2024, 452:139555.
Molecules.2024, 29(6):1392.
Pest Manag Sci.2019, 75(9):2530-2541
Development.2024, 151(20):dev202518.
Br J Pharmacol.2020, 10.1111
Front Immunol.2018, 9:2655
Current Topics in Nutraceutical Research2021, 19(1),p90-105.
Acta horticulturae2017, 1158:257-268
Oxid Med Cell Longev.2020, 2020:8887251.
Biomolecules.2024, 14(10):1257.
Related and Featured Products
Chemosphere. 2003 Feb;50(6):807-11.
The role of citric acid on the phytoremediation of heavy metal contaminated soil.[Pubmed:
12688495]
Adsorption and hydroponics experiments were conducted to study the role of Citric acid on the phytoremediation of heavy metal contaminated soil.
METHODS AND RESULTS:
The results show that addition of Citric acid decreased the adsorption of both lead and cadmium, such an effect was bigger for cadmium than for lead. The decrease in the adsorption of Pb and Cd was mainly due to a decrease of pH in the presence of Citric acid. The presence of Citric acid could alleviate the toxicity of Pb and Cd to radish, and stimulate their transportation from root to shoot. The studies of heavy metal forms using sequential extraction demonstrated that lead was mainly existed as FHAC (a lower bioavailable form) in the root, while F(HCl) was the dominant form in the leaf. The addition of Citric acid to the soil changed the concentration and relative abundance of all the forms. The detoxifying effect of Citric acid to Pb in shoots might result from the transformation of higher toxic forms into lower toxic forms. Cadmium was mainly present as F(NaCl), therefore, it had higher toxicity than lead.
CONCLUSIONS:
The addition of Citric acid increased the abundance of F(H2O) + F(NaCl), indicating that Citric acid treatment could transform cadmium into more transportable forms.
BMC Oral Health. 2014 Jun 23;14:77.
Efficacy of citric acid denture cleanser on the Candida albicans biofilm formed on poly(methyl methacrylate): effects on residual biofilm and recolonization process.[Pubmed:
24957210]
It is well known that the use of denture cleansers can reduce Candida albicans biofilm accumulation; however, the efficacy of Citric acid denture cleansers is uncertain. In addition, the long-term efficacy of this denture cleanser is not well established, and their effect on residual biofilms is unknown. This in vitro study evaluated the efficacy of Citric acid denture cleanser treatment on C. albicans biofilm recolonization on poly(methyl methacrylate) (PMMA) surface.
METHODS AND RESULTS:
C. albicans biofilms were developed for 72 h on PMMA resin specimens (n = 168), which were randomly assigned to 1 of 3 cleansing treatments (CTs) overnight (8 h). CTs included purified water as a control (CTC) and two experimental groups that used either a 1:5 dilution of Citric acid denture cleanser (CT5) or a 1:8 dilution of Citric acid denture cleanser (CT8). Residual biofilms adhering to the specimens were collected and quantified at two time points: immediately after CTs (ICT) and after cleaning and residual biofilm recolonization (RT). Residual biofilms were analyzed by quantifying the viable cells (CFU/mL), and biofilm architecture was evaluated by confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM). Denture cleanser treatments and evaluation periods were considered study factors. Data were analyzed using two-way ANOVA and Tukey's Honestly Significant Difference (HSD) test (α = 0.05).
Immediately after treatments, Citric acid denture cleansing solutions (CT5 and CT8) reduced the number of viable cells as compared with the control (p < 0.01). However, after 48 h, both CT groups (CT5 and CT8) showed biofilm recolonization (p < 0.01). Residual biofilm recolonization was also detected by CLSM and SEM analysis, which revealed a higher biomass and average biofilm thickness for the CT8 group (p < 0.01).
CONCLUSIONS:
Citric acid denture cleansers can reduce C. albicans biofilm accumulation and cell viability. However, this CT did not prevent biofilm recolonization.
Iranian Journal of Fisheries Sciences, 2010, 9(9):279-292.
Inhibitory impacts of natural antioxidants (ascorbic and citric acid) and vacuum packaging on lipid oxidation in frozen Persian sturgeon fillets[Reference:
WebLink]
This study was aimed to investigate effects of aqueous Citric acid (CA) and ascorbic acid (AA)
on lipid oxidation in comparison with effect of vacuum packaging in order to find better
treatment to delay improper changes in the Persian sturgeon (Acipenser persicus) fillets during
frozen storage due to lipid oxidation.
METHODS AND RESULTS:
In this study traditional packaging, vacuum packaging,
ascorbic acid solution (0.5 %) and Citric acid solution (0.5 %) were considered as treatments.
Rancidity development was measured by several biochemical indicators including Free Fatty
Acids, Peroxide values and Thiobarbituric acid. Also pH, expressible moisture and sensory
properties were measured during 6 months storage. Results showed that free fatty acid (FFA),
primary and secondary oxidation products of control samples were significantly higher than
those in other treatments (p<0.05). Also, expressible moisture and pH value of treated samples
were significantly lower than those in control (p<0.05). However both antioxidants (AA and CA)
extended shelf life of frozen fillets but rancidity development in CA treated samples was higher
than other samples during storage.
CONCLUSIONS:
Results showed that all three treatments had significant effect
on delaying lipid oxidation (p<0.05) but usage of AA and vacuum packaging had the best effect
on delaying lipid oxidation and increasing shelf-life of fillets (p<0.05). Thus the employment of
AA and vacuum packaging alone or in combination with other protective strategies is
recommended.
J Med Food. 2014 May;17(5):588-98.
Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice.[Pubmed:
24433072]
Citric acid is a weak organic acid found in the greatest amounts in citrus fruits. This study examined the effect of Citric acid on endotoxin-induced oxidative stress of the brain and liver.
METHODS AND RESULTS:
Mice were challenged with a single intraperitoneal dose of lipopolysaccharide (LPS; 200 μg/kg). Citric acid was given orally at 1, 2, or 4 g/kg at time of endotoxin injection and mice were euthanized 4 h later. LPS induced oxidative stress in the brain and liver tissue, resulting in marked increase in lipid peroxidation (malondialdehyde [MDA]) and nitrite, while significantly decreasing reduced glutathione, glutathione peroxidase (GPx), and paraoxonase 1 (PON1) activity. Tumor necrosis factor-alpha (TNF-α) showed a pronounced increase in brain tissue after endotoxin injection. The administration of Citric acid (1-2 g/kg) attenuated LPS-induced elevations in brain MDA, nitrite, TNF-α, GPx, and PON1 activity. In the liver, nitrite was decreased by 1 g/kg Citric acid. GPx activity was increased, while PON1 activity was decreased by Citric acid. The LPS-induced liver injury, DNA fragmentation, serum transaminase elevations, caspase-3, and inducible nitric oxide synthase expression were attenuated by 1-2 g/kg Citric acid. DNA fragmentation, however, increased after 4 g/kg Citric acid.
CONCLUSIONS:
Thus in this model of systemic inflammation, Citric acid (1-2 g/kg) decreased brain lipid peroxidation and inflammation, liver damage, and DNA fragmentation.
J Anim Sci. 2000 Mar;78(3):682-9.
The effects of citric acid on phytate-phosphorus utilization in young chicks and pigs.[Pubmed:
10764076]
Several bioassays were conducted with young chicks and pigs fed phosphorus (P)-deficient corn-soybean meal diets.
METHODS AND RESULTS:
With diets for chicks containing .62% Ca and .42% P (.10% available P), graded doses of a Citric acid + sodium citrate (1:1, wt:wt) mixture (0, 1, 2, 4, or 6% of diet) resulted in linear (P < .01) increases in both weight gain and tibia ash. Relative to chicks fed no Citric acid, tibia ash (%) and weight gain (g/d) were increased by 43 and 22%, respectively, in chicks fed 6% Citric acid. Additional chick trials showed that 6% Citric acid alone or sodium citrate alone was as efficacious as the Citric acid + sodium citrate mixture and that 1,450 U/kg of phytase produced a positive response in bone ash and weight gain in chicks fed a diet containing 6% citrate. Varying the Ca:available P ratio with and without citrate supplementation indicated that Citric acid primarily affected phytate-P utilization, not Ca, in chicks. Moreover, chicks did not respond to citrate supplementation when fed a P-deficient (.13% available P), phytate-free casein-dextrose diet. Young pigs averaging 10 to 11 kg also were used to evaluate Citric acid efficacy in two experiments. A P-deficient corn-soybean meal basal diet was used to construct five treatment diets that contained 1) no additive, 2) 3% Citric acid, 3) 6% Citric acid, 4) 1,450 U/kg phytase, and 5) 6% Citric acid + 1,450 U/kg phytase. Phytase supplementation increased (P < .01) weight gain, gain:feed, and metatarsal ash, whereas Citric acid addition increased only gain:feed (P < .05) and metatarsal ash (P < .08). A subsequent 22-d pig experiment was conducted to evaluate the effect of lower levels of Citric acid (0, 1, 2, or 3%) or 1,450 U/kg phytase addition to a P-deficient corn-soybean meal diet. Phytase supplementation improved (P < .01) all criteria measured. Weight gain and gain:feed data suggested a response to Citric acid addition, but this was not supported by fibula ash results (P > .10). The positive responses to phytase were much greater than those to Citric acid in both pig experiments.
CONCLUSIONS:
Thus, dietary Citric acid effectively improved phytate P utilization in chicks but had a much smaller effect in pigs.