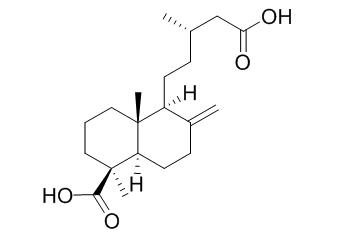
Junicedric acid
Junicedric acid has gastroprotective effects.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Planta Med.2022, 88(9-10):794-804.
Phytochem Anal.2022, doi: 10.1002
J Insect Sci.2020, 20(5):18.
Trop J Nat Prod Res.2019, 3(1):6-9
Molecules.2021, 26(9):2791.
Int J Mol Sci.2023, 25(1):283.
The Journal of Animal & Plant Sciences.2020, 30(6):1366-1373
Food Chem.2016, 191:81-90
Front Pharmacol.2022, 13:972825.
J.Pharm. & Biome. Anal.2023, 2: 100018.
Related and Featured Products
Molecules. 2011 Oct 13;16(10):8614-28.
Synthesis and pharmacological activity of diterpenylnaphthoquinone derivatives.[Pubmed:
21996716]
METHODS AND RESULTS:
New diterpenylquinones, combining a diterpene diacid and a naphthoquinone, were prepared from Junicedric acid and lapachol. The new derivatives were assessed as gastroprotective agents by the HCl-EtOH-induced gastric lesions model in mice as well as for basal cytotoxicity on the following human cell lines: Normal lung fibroblasts (MRC-5), gastric epithelial adenocarcinoma (AGS), and hepatocellular carcinoma (Hep G2).
CONCLUSIONS:
Several of the new compounds were significantly active as antiulcer agents and showed selective cytotoxicity against AGS cells.
Molecules. 2010 Oct 21;15(10):7378-94.
Synthesis, gastroprotective effect and cytotoxicity of new amino acid diterpene monoamides and diamides.[Pubmed:
20966879 ]
Following our studies on the gastroprotective effect and cytotoxicity of terpene derivatives, new amides were prepared from the diterpene 8(17)-labden-15,19-dioic acid (Junicedric acid) and its 8(9)-en isomer with C-protected amino acids (amino acid esters).
METHODS AND RESULTS:
The new compounds were evaluated for their gastroprotective effect in the ethanol/HCl-induced gastric lesions model in mice, as well as for cytotoxicity using the following human cell lines: normal lung fibroblasts (MRC-5), gastric adenocarcinoma cells (AGS) and liver hepatocellular carcinoma (Hep G2). A dose-response experiment showed that at 25 mg/kg the C-15 leucyl and C-15,19-dileucylester amides of Junicedric acid reduced gastric lesions by about 65.6 and 49.6%, respectively, with an effect comparable to lansoprazole at 20 mg/kg (79.3% lesion reduction). The comparison of the gastroprotective effect of 18 new amino acid ester amides was carried out at a single oral dose of 25 mg/kg. Several compounds presented a strong gastroprotective effect, reducing gastric lesions in the 70.9-87.8% range.
CONCLUSIONS:
The diprolyl derivative of Junicedric acid, the most active product of this study (87.8% lesion reduction at 25 mg/kg) presented a cytotoxicity value comparable with that of the reference compound lansoprazole. The structure-activity relationships are discussed.
Helvetica Chimica Acta, 2010, 91(5):856-861.
Terpenoids from Two Dicranopteris Species.[Reference:
WebLink]
METHODS AND RESULTS:
Two new compounds, (6S,13S)-6-{[β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl]oxy}cleroda-3,14-dien-13-ol (1) and kadsuric acid 3-methyl ester (2), together with nine known compounds, (6S,13E)-6-{[β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl]oxy}cleroda-3,13-dien-15-ol (3), (6S,13S)-6-[6-O-acetyl-β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl]oxy}-13-{[α-L-rhamnopyranosyl-(1→4)-β-D-fucopyranosyl]oxy}cleroda-3,14-diene (4), (6S,13S)-6-{[6-O-β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl]oxy}-13-{[α-L-rhamnopyranosyl-(1→4)-β-D-fucopyranosyl]oxy}cleroda-3,14-diene (5), 15-hydroxydehydroabietic acid (6), 15-hydroxylabd-8(17)-en-19-oic acid (7), Junicedric acid (8), (4β)-kaur-16-en-18-oic acid (9), (4β)-16-hydroxykauran-18-oic acid (10), and (4β,16β)-16-hydroxykauran-18-oic acid (11) were isolated from the fronds of Dicranopteris linearis or D. ampla.
CONCLUSIONS:
Their structures were established by extensive 1D- and 2D-NMR spectroscopy. Compounds 1 and 3–8 showed no anti-HIV activities.