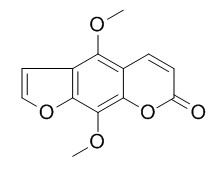
Isopimpinellin
Isopimpinellin has chemopreventive effects, it effectively inhibits mouse COH activity (IC50 values 19-40 microM).Isopimpinellin is a new inhibitor against the Leishmania APRT enzyme.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biochem Biophys Res Commun.2018, 505(1):194-200
Food Chemistry2023, 137837.
Food Analytical Methods2020, 1-10
Pharmacol Rep.2019, 71(2):289-298
Sci Rep.2024, 14(1):28864.
Front Pharmacol.2025, 16:1611342.
Anal Chim Acta.2021, 1180:338874.
Int J Mol Sci.2024, 25(2):764.
Phytomedicine.2015, 22(4):498-503
Foods.2022, 12(1):136.
Related and Featured Products
Photochem Photobiol. 1996 Mar;63(3):306-7.
Isopimpinellin is not phototoxic in a chick skin assay.[Pubmed:
8881335]
METHODS AND RESULTS:
Synthetic Isopimpinellin (5,8-dimethoxypsoralen), confirmed to contain as impurities only trace quantities at most of psoralen, bergapten (5-methoxypsoralen) and xanthotoxin (8-methoxypsoralen), is not phototoxic when tested in a chick skin bioassay system. These findings are at variance with earlier studies showing Isopimpinellin to be phototoxic against chick skin and support the conclusion that Isopimpinellin is photobiologically inactive.
CONCLUSIONS:
As recently proposed by others, the several reports of Isopimpinellin photoactivity are most likely attributable to contamination by small amounts of highly active psoralens such as bergapten or xanthotoxin.
Planta Med. 1987 Jun;53(3):306-7.
Isopimpinellin is not phototoxic to viruses and cells.[Pubmed:
3628561 ]
Isopimpinellin is not phototoxic to viruses and cells.
Acta Crystallographica, 2003, 59(10):o1506–8.
Redetermination and comparative structural study of isopimpinellin: A new inhibitor against the Leishmania APRT enzyme[Reference:
WebLink]
The title compound (Isopimpinellin,alternative name 5,8-dimethoxypsoralen), C13H10O5, is a natural product extracted from Adiscanthus fusciflorus (Rutaceae).
METHODS AND RESULTS:
Our biochemical tests show that this compound has inhibitory activity against the enzyme adenine phosphoribosyltransferase (APRT) from Leishmania, a tropical parasite causing endemic disease in poor countries. It crystallizes in the centrosymmetric space group P2(1)/c, with one molecule in the asymmetric unit, and has at least two C-H...O intermolecular interactions, leading to the formation of centrosymmetric dimers.
Carcinogenesis. 2002 Oct;23(10):1667-75.
Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice.[Pubmed:
12376476]
The current study was designed to evaluate the effects of oral administration of the citrus coumarin, Isopimpinellin, on skin tumor initiation by topically applied benzo[a]pyrene (B[a]P) and 7,12-dimethylbenz[a]anthracene (DMBA).
METHODS AND RESULTS:
To evaluate the effects of orally administered Isopimpinellin on skin tumor initiation by B[a]P and DMBA, its effects on DNA adduct formation were first evaluated. Female SENCAR mice were pre-treated twice with corn oil, or Isopimpinellin (70 mg/kg body wt per os) at 24 h and 2 h prior to topical treatment with B[a]P or DMBA. Another citrus coumarin, imperatorin, was also included in these experiments for comparison. Orally administered Isopimpinellin and imperatorin significantly inhibited B[a]P-DNA adduct formation by 37 and 26%, respectively. Imperatorin also blocked DMBA-DNA adduct formation by 43%. In a second dose-response study, orally administered Isopimpinellin (35, 70 and 150 mg/kg) blocked DMBA-DNA adduct formation by 23, 56 and 69%, respectively. For the tumor study, mice were pretreated orally with corn oil or Isopimpinellin at 24 and 2 h prior to initiation with DMBA, and 2 weeks later promotion began with 12-O-tetradecanoylphorbol-13-acetate (TPA). Isopimpinellin significantly reduced the mean number of papillomas per mouse by 49, 73 and 78% compared to corn oil controls at 30, 70 and 150 mg/kg body wt, respectively. Orally administered Isopimpinellin also significantly reduced the percentage of mice with papillomas at the highest dose tested (150 mg/kg). The effectiveness of Isopimpinellin given topically over a broad dose range against DMBA tumor initiation was also evaluated for comparison. As part of this study, several parameters of systemic toxicity were evaluated following oral dosing with Isopimpinellin and imperatorin. Mice were treated orally with corn oil, Isopimpinellin or imperatorin (35, 70 and 150 mg/kg body wt per os) once daily for four consecutive days, killed at 24 h after the last dose, and livers, lungs, and kidneys evaluated histologically. In addition, urinary parameters of nephrotoxicity, blood parameters of liver and kidney function, and thrombin clotting time were assayed. No significant changes in blood clotting, or renal or hepatic function were observed. There was, however, a significant increase in liver wt accompanied by cytoplasmic vacuolation of hepatocytes. There were no histopathological changes in lungs or kidneys.
CONCLUSIONS:
Overall, these data indicate that Isopimpinellin (and imperatorin) have chemopreventive effects when administered orally on skin tumor initiation by DMBA.
J Oleo Sci. 2011;60(11):575-8.
Microbial transformation of Isopimpinellin by Glomerella cingulata.[Pubmed:
22027023]
METHODS AND RESULTS:
Microbial transformation studies conducted on Isopimpinellin (1) by the fungus Glomerella cingulata have revealed that 1 was metabolized to give the corresponding reduced acid, 5,8-dimethoxy-6,7-furano-hydrocoumaric acid (2). The structure of metabolite 2 was elucidated by high-resolution mass spectrometry (HR-MS), extensive NMR techniques, including (1)H NMR, (13)C NMR, (1)H-(1)H correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC) and heteonuclear multiple bond coherence (HMBC).
CONCLUSIONS:
The biotransformed product 2 showed weak a in vitro β-secretase (BACE1) inhibitory effect.