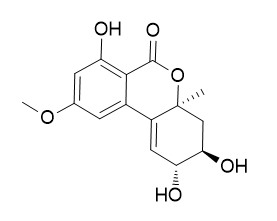
Isoaltenuene
Isoaltenuene shows antibiotic activity against Gram-positive bacteria; it also shows a minor phytotoxic activity on tomato leaves at level of 20 ug/spot . Isoaltenuene exhibits cytotoxic activity against lung cancer cell line A549, breast cancer cell line MDA-MB-231 and pancreatic cancer cell line PANC-1.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmaceutics.2023, 15(6):1771.
Aging (Albany NY).2021, 13(19):22867-22882.
Front Plant Sci.2021, 12:673337.
Horticulture, Environment, and Biotechnology2025, 66:729-739.
Food Chem.2024, 458:140201.
Pharmaceutics.2020, 12(9):845.
Inflammation.2020, 43(5):1716-1728.
Pathogens.2018, 7(3):E62
Microchemical Journal2023, 194:109249
African J. Agricultural Research 2017, 12(13):1164-1168
Related and Featured Products
Yao Xue Xue Bao. 2013 Jun;48(6):891-5.
A new sesquiterpenoid from fungus Colletotrichum sp. and its cytotoxicity.[Pubmed:
23984524]
METHODS AND RESULTS:
A novel sesquiterpenoid (1) and three known compounds identified as Isoaltenuene (2), altenuene (3), and alternariol 4, 10-O-dimethyl ether (4), were isolated in our investigation of the cytotoxic constituents from solid cultures of the endophytic fungus Colletotrichum sp. The structures of these compounds were elucidated through spectroscopic data analysis.
CONCLUSIONS:
All compounds exhibited cytotoxic activity against lung cancer cell line A549, breast cancer cell line MDA-MB-231 and pancreatic cancer cell line PANC-1. Compound 4 could induce the PANC-1 cells inflation or death, but couldn't induce apoptosis at the IC50 of 60.2 microg x mL(-1).
Mycotoxin Res. 1989 Sep;5(2):69-76.
Isolation and structure elucidation of isoaltenuene, a new metabolite ofAlternaria alternata.[Pubmed:
23605299 ]
Isoaltenuene, a previously unknownAlternaria metabolite has been isolated from a rice culture ofAlternaria alternata and purified by semipreparative HPLC.
METHODS AND RESULTS:
The assigned structure, elucidated by UV, IR, MS, and NMR spectroscopy, was 2', 3', 4', 5'-tetrahydro-3, 4'β, 5'α-trihydroxy-5-methoxy-2'α-methyldibenzo (α)-pyrone and corresponded to a diasteroisomer of altenuene with inverted configuration at C-2'.
CONCLUSIONS:
Isoaltenuene showed a minor phytotoxic activity on tomato leaves at level of 20 μg/spot and no antifungal activity onGeotrichum candidum (up to 20 μg/disk).
Nat Prod Res. 2015;29(9):848-52
A new alternariol glucoside from fungus Alternaria alternate cib-137.[Pubmed:
25520187 ]
METHODS AND RESULTS:
A new secondary metabolite, 2-O-methylalternariol 4-O-β-[4-methoxyl-glucopyranoside] (1), together with four known compounds alternariol methyl ether (2), altenuene (3), Isoaltenuene (4) and 2-(2'S-hydroxypropyl)-5-methyl-7-hydroxychromone (5), was isolated from the fungus Alternaria alternate cib-137. Its structure was elucidated on the basis of spectroscopic data.
CONCLUSIONS:
Compounds 3 and 4 demonstrated moderate activity against Staphylococcus aureus.
J Nat Prod. 2006 Apr;69(4):612-5.
Altenuene derivatives from an unidentified freshwater fungus in the family Tubeufiaceae.[Pubmed:
16643037 ]
METHODS AND RESULTS:
Four new altenuene derivatives called dihydroaltenuenes A (1) and B (2) and dehydroaltenuenes A (3) and B (4), along with five known compounds, including Isoaltenuene (5), altenuene (6), and 5'-epialtenuene (7), were isolated from cultures of an unidentified freshwater aquatic fungal species in the family Tubeufiaceae. The structures of 1-4 were determined by analysis of NMR and MS data.
The relative stereochemistry was determined on the basis of (1)H NMR J-values and NOE data, while the absolute configuration of a representative member of the group (5) was assigned by CD spectral analysis of its bis-N,N-dimethylaminobenzoate derivative.
CONCLUSIONS:
Compounds 1, 3, 4, 5, and 6 showed antibiotic activity against Gram-positive bacteria.
Gymnoside III
Catalog No: CFN95033
CAS No: 899430-03-6
Price: $238/10mg
Cordifolioside A
Catalog No: CFN95040
CAS No: 155179-20-7
Price: $318/10mg
5,7,2',4'-Tetrahydroxy-8,3'-di(gamma,gamma-dimethylallyl)-isoflavanone
Catalog No: CFN95084
CAS No: 141846-47-1
Price: $413/5mg
Nardoaristolone B
Catalog No: CFN95203
CAS No: 1422517-82-5
Price: $398/5mg
Isosaponarin 2''-O-glucoside (Isovitexin-2''-4'-di-O-beta-D-glucoside)
Catalog No: CFN95296
CAS No: 63316-27-8
Price: $318/10mg
Deoxy euphorbia factor L1
Catalog No: CFN95340
CAS No: 247099-01-0
Price: $318/10mg
4'-Hydroxy-3',5,5',6,7,8-hexamethoxyflavone
Catalog No: CFN95407
CAS No: 85644-03-7
Price: $318/5mg
erythro-Austrobailignan-6
Catalog No: CFN95501
CAS No: 114127-24-1
Price: $318/10mg
(3,4-Dihydroxyphenyl)methyl 3-(beta-D-glucopyranosyloxy)-4-hydroxybenzoate
Catalog No: CFN95531
CAS No: 877461-90-0
Price: $318/10mg
12-Acetoxy ganoderic acid D
Catalog No: CFN95535
CAS No: N/A
Price: $318/5mg