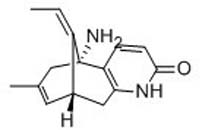
(-)-Huperzine A
(-)-Huperzine A is a naturally occurring potent reversible AChE inhibitor that penetrates the blood-brain barrier, it also has several neuroprotective effects including modification of beta-amyloid peptide, reduction of oxidative stress, anti-inflammatory, anti-apoptotic and induction and regulation of nerve growth factor.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
National Natural Science Foundation of China2024, pages 33.
Molecules 2021, 26(4),1092.
Evid Based Complement Alternat Med.2017, 2017:9764843
Heliyon2020, 6(6):e04337.
Phytochemistry2018, 15:83-92
Molecules.2024, 29(21):5161.
Oxid Med Cell Longev.2022, 2022:5888636.
Foods.2021, 10(11):2754.
J Sep Sci.2021, 44(22):4064-4081.
Processes2021, 9(5),831.
Related and Featured Products
Chem Biol Interact. 2013 Mar 25;203(1):120-4.
A combination of [+] and [-]-Huperzine A improves protection against soman toxicity compared to [+]-Huperzine A in guinea pigs.[Pubmed:
23123250]
The neuropathologic mechanisms after exposure to lethal doses of nerve agent are complex and involve multiple biochemical pathways. Effective treatment requires drugs that can simultaneously protect by reversible binding to the acetylcholinesterase (AChE) and blocking cascades of seizure related brain damage, inflammation, neuronal degeneration as well as promoting induction of neuroregeneration. (-)-Huperzine A ([-]-Hup A), is a naturally occurring potent reversible AChE inhibitor that penetrates the blood-brain barrier. It also has several neuroprotective effects including modification of beta-amyloid peptide, reduction of oxidative stress, anti-inflammatory, anti-apoptotic and induction and regulation of nerve growth factor.
METHODS AND RESULTS:
Toxicities at higher doses restrict the neuroporotective ability of (-)-Huperzine A for treatment. The synthetic stereoisomer, [+]-Hup A, is less toxic due to poor AChE inhibition and is suitable for both pre-/post-exposure treatments of nerve agent toxicity. [+]-Hup A block the N-methyl-D-aspartate (NMDA)-induced seizure in rats, reduce excitatory amino acid induced neurotoxicity and also prevent soman induced toxicity with minimum performance decrement. Unique combinations of two stereo-isomers of Hup A may provide an excellent pre/post-treatment drug for the nerve agent induced seizure/status epilepticus. We investigated a combination of [+]-Hup A with a small dose of (-)-Huperzine A ([+] and (-)-Huperzine A) against soman toxicity. Our data showed that pretreatment with a combination [+] and (-)-Huperzine A significantly increased the survival rate and reduced behavioral abnormalities after exposure to 1.2 × LD(50) soman compared to [+]-Hup A in guinea pigs. In addition, [+] and (-)-Huperzine A pretreatment inhibited the development of high power of EEG better than [+]-Hup A pretreatment alone.
CONCLUSIONS:
These data suggest that a combination of [+] and (-)-Huperzine A offers better protection than [+]-Hup A and serves as a potent medical countermeasure against lethal dose nerve agent toxicity in guinea pigs.
Org Lett. 2013 Feb 15;15(4):882-5.
A novel synthesis of (-)-huperzine A via tandem intramolecular aza-Prins cyclization-cyclobutane fragmentation.[Pubmed:
23346936]
The acetylcholinesterase inhibitor (-)-Huperzine A was synthesized from (S)-4-hydroxycyclohex-2-enone in 17 steps by a route that involved two cyclobutane fragmentations. The first of these employed a retro-aldol cleavage to generate the α-pyridone ring of huperzine A, and the second invoked a novel intramolecular aza-Prins reaction in tandem with stereocontrolled scission of a cyclobutylcarbinyl cation to create the aminobicyclo[3.3.1]nonene framework of the natural alkaloid.
Org Lett. 2012 Sep 7;14(17):4446-9.
An efficient total synthesis of (-)-huperzine A.[Pubmed:
22900755]
The total synthesis of Lycopodium alkaloid (-)-Huperzine A has been accomplished in 10 steps with 17% overall yield from commercially abundant (R)-pulegone. The synthetic route features an efficient synthesis of 4 via a Buchwald-Hartwig coupling reaction, a dianion-mediated highly stereoselective alkylation of 4, and a rare example of an intramolecular Heck reaction of an enamine-type substrate. The stereoselective β-elimination and the accompanying Wagner-Meerwein rearrangement are of particular interest.