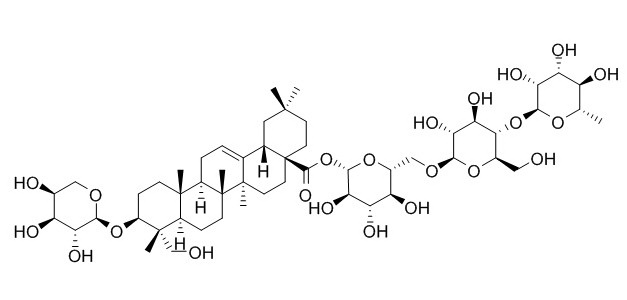
Hederacoside D
Hederacoside D is a narural product from Hedera nepalensis.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Adv Healthc Mater.2024, 13(13):e2303276.
J. Traditional Thai Medical Res. 2022,8(1):1-14.
Appl. Sci. 2021, 11(10),4666.
Front Pharmacol.2021, 12:762829.
J Mol Med (Berl).2018, 96(7):661-672
Neurotoxicology.2022, 91:218-227.
Arabian Journal of Chemistry2024, 17(3):105648
Toxicol In Vitro.2022, 81:105346.
European Journal of Integrative Medicine2018, 20:165-172
Eur J Pharmacol.2024, 978:176749.
Related and Featured Products
J Sep Sci. 2016 Sep;39(17):3292-301.
Pharmacokinetic parameters of three active ingredients hederacoside C, hederacoside D, and ɑ-hederin in Hedera helix in rats.[Pubmed:
27377040 ]
In Hedera helix hederacoside C, Hederacoside D, and ɑ-hederin are three major bioactive saponins and play pivotal roles in the overall biological activity.
METHODS AND RESULTS:
In this study, a specific and sensitive ultra-high performance liquid chromatography with tandem mass spectrometry method has been developed and validated for the quantification of three major bioactive saponins in rat plasma. Chromatographic separation was performed on a reversed-phase Thermo Hypersil GOLD C18 column (2.1 mm × 50 mm, 1.9 μm) using a gradient mobile phase system of acetonitrile-water containing 0.1% formic acid. The assay was successfully applied to study the pharmacokinetic behavior of the three analytes in rats after oral and intravenous administration of a mixture of saponins (hederacoside C, Hederacoside D, and ɑ-hederin). Further research was performed to compare the pharmacokinetic behavior of the three analytes after the oral administration of a mixture of saponins and an extract of saponins from Hedera helix, and results showed that double peaks were evident on concentration-time profile for each of the three saponins.
CONCLUSIONS:
The difference in the pharmacokinetic characteristics of three saponins between a mixture of saponins and an extract of saponins from Hedera helix was found in rat, which would be beneficial for the preclinical research and clinical use of Hedera helix.